Abstract
Group 1 CD1 (CD1a, -b, and -c) presents self and foreign lipid antigens to multiple T-cell subsets in humans. However, in the absence of a suitable animal model, the specific functions and developmental requirements of these T cells remain unknown. To study group 1 CD1-restricted T cells in vivo, we generated double transgenic mice (HJ1Tg/hCD1Tg) that express group 1 CD1 molecules in a similar pattern to that observed in humans (hCD1Tg) as well as a TCR derived from a CD1b-autoreactive T-cell line (HJ1Tg). Using this model, we found that similar to CD1d-restricted NKT cells, HJ1 T cells exhibit an activated phenotype (CD44hiCD69+CD122+) and a subset of HJ1 T cells expresses NK1.1 and is selected by CD1b-expressing hematopoietic cells. HJ1 T cells secrete proinflammatory cytokines in response to stimulation with CD1b-expressing dendritic cells derived from humans as well as hCD1Tg mice, suggesting that they recognize species conserved self-lipid antigen(s). Importantly, this basal autoreactivity is enhanced by TLR-mediated signaling and HJ1 T cells can be activated and confer protection against Listeria infection. Taken together, our data indicate that CD1b-autoreactive T cells, unlike mycobacterial lipid antigen-specific T cells, are innate-like T cells that may contribute to early anti-microbial host defense.
Introduction
The CD1 antigen-presenting molecules are similar in structure to MHC class I, but are specialized to present lipid antigens to T cells.1 These antigens include mammalian self-lipids and foreign lipids derived from specific microorganisms that are loaded onto CD1 in the endosomal compartments of the cell.2-6 Five members of the CD1 family have been identified and can be classified into 3 groups based on sequence homology.7 Group 1 CD1 (CD1a, b, and c) and group 2 CD1 (CD1d) are expressed on the cell surface and act as antigen-presenting molecules, while CD1e acts as a chaperone to facilitate lipid delivery onto CD1b and CD1d molecules.8 While humans express all CD1 isoforms, muroid rodents only express CD1d.1
To date, CD1d has been the most extensively studied member of the CD1 family. CD1d presents lipid antigens to a unique subset of T cells, NKT cells. The best-known subset of CD1d-restricted NKT cells uses an invariant TCRα chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans); they are therefore referred to as invariant NKT (iNKT) cells.9 Unlike most conventional T cells, iNKT cells exhibit an activated phenotype which is partly the results of their positive selection being mediated by CD1d-expressing thymocytes, instead of thymic epithelial cells.10 iNKT cells rapidly secrete IFN-γ, IL-4, and other cytokines on TCR stimulation.9,11,12 Activated iNKT cells in turn activate DC, macrophages and NK cells and thereby impact subsequent B and T-cell responses.13 Therefore, iNKT cells play a critical role in bridging innate and adaptive immune responses.
In contrast to iNKT cells, our knowledge regarding group 1 CD1-restricted T cells is largely limited to the observation of long-term cultured T-cell clones, as a suitable animal model has only recently been developed.14 Group 1 CD1-restricted T-cell lines have been isolated from human patients infected with Mycobacterium tuberculosis and Mycobacterium leprae. These T-cell lines express TCRαβ and belong to the DN, CD4+ and CD8+ T-cell subsets.3,15-18 Some of these T-cell lines respond to lipid antigens derived from the mycobacterial cell wall, suggesting that group 1 CD1-restricted T cells are involved in anti-mycobacterial immune responses.3,6,15,17,19 Interestingly, the majority of the group 1 CD1-restricted human T-cell lines are autoreactive, indicating that autoreactivity may be a key feature of a substantial fraction of group 1 CD1-resticted T cells.2,4,20-22 Indeed, a recent study showed that CD1a-autoreactive T cells could be readily detected in human peripheral blood.23 It has been shown that autoreactive group 1 CD1-restricted T-cell lines exhibit enhanced responses in the presence of TLR agonists or bacteria, indicating that these T cells may also be activated during infection.2,24 However, much regarding the developmental requirements and physiologic role of these autoreactive group 1 CD1-restricted T cells is still unknown.
It has been reported that the TCR repertoire of human group 1 CD1-restricted T cells is highly diverse,2,3,23 which is supported by our own studies of T-cell lines derived from mice transgenic for the group 1 CD1 molecules (hCD1Tg mice).14 These transgenic mice express group 1 CD1 in a pattern mimicking that observed in humans and support the development of group 1 CD1-restricted T cells. Similar to human group 1 CD1-restricted T-cell lines, the majority of the group 1 CD1-restricted T-cell lines isolated from hCD1Tg mice are autoreactive, produce IFN-γ on stimulation and specifically lyse group 1 CD1-expressing targets. One such T-cell line is CD1b-autoreactive HJ1. To facilitate the study of these autoreactive group 1 CD1-restricted T cells in vivo, we generated a transgenic mouse that expresses the HJ1 TCR (HJ1Tg) and then crossed it onto the hCD1Tg background to generate HJ1Tg/hCD1Tg mice.
We found that the HJ1 T cells isolated from HJ1Tg/hCD1Tg mice maintain the CD1b-autoreactivity and exhibit an activated phenotype even in the thymus. A subset of HJ1 T cells expresses NK1.1 and is enriched in liver. Similar to iNKT cells, this T-cell population is positively selected by CD1b-expressing hematopoietic cells. On stimulation with CD1b-expressing DC, HJ1 T cells produce substantial amounts of proinflammatory cytokines. The addition of bacteria or TLR agonists induces DC to produce IL-12/IL-23, which synergistically enhances the autoreactivity of HJ1 T cells. Moreover, the adoptive transfer of HJ1 T cells protects recipient mice against listerial infection. Altogether, our data demonstrate that both the developmental requirements and functions of CD1b-autoreactive HJ1 T cells resemble those of iNKT cells and could in turn be manipulated for use in anti-microbial therapies.
Methods
Generation of HJ1Tg mice
To generate HJ1Tg mice, the variable regions of TCR genes were amplified from HJ1 CTL genomic DNA using the following primer pairs: Vα8.5 forward-5′-TCCCCCCGGGCTTCTCACTGCCTAGCCATGCGTCCTGGCACCTGCTCA-3′ and Jα14 reverse-5′-ATAAGAATGCCGCGGCAGCCATGGTTCAGGTCCAAATACT-3′, and Vβ2 forward-5′-CCGCTCGAGAGGAAGCATGTGGCAGTTTTGCATTCTGTGC-3′ and Jβ2.3 reverse-5′-TCCCCGCGGCCCCTTCATTACAGCTCCCAACTTAC-3′. Amplified TCRα and β DNA fragments were cloned into pTα and pTβ vector,25 respectively. TCR constructs were coinjected into fertilized B6 oocytes. Potential founders were screened for TCRα transgene by PCR using the following primers: forward 5′-TGACACCTGCTCAGTTCTTGTGC-3′ and reverse 5′-TAGCTTGTTCCCTGCACTTGG-3′, and for TCRβ expression by staining with anti-Vβ2 mAb. Two transgenic lines were established; the offspring of 1 line expressed both TCR transgenes and were further crossed onto hCD1Tg (line 78 hCD1 Tg mice express CD1b and CD1c),14 Rag2−/− and CD1d−/−26 backgrounds.
Mice
CD45.1 congenic B6, IFNγ−/− and Rag−/− mice were purchased from The Jackson Laboratory. hCD1Tg mice were crossed onto CD45.1 congenic B6 and IFNγ−/− backgrounds for this study. All animals were housed in specific pathogen-free facilities at the University of Chicago and Northwestern University, and all animal studies were approved by their respective Institutional Animal Care and Use Committees.
Primary cell preparations and dendritic cell generation
Thymocytes, splenocytes, and hepatic leukocytes were isolated as described previously.14 HJ1 T cells were purified from the spleen or liver of HJ1Tg/hCD1Tg/Rag−/− mice through depletion of CD11b+ and MHC II+ cells using a magnetized MS column (Miltenyi, > 90% TCRβ+). Bone marrow–derived DC (BMDC) were prepared using GM-CSF and IL-4 as described.27 Human monocyte-derived DC were prepared by culturing adherent cells from peripheral blood leukocytes in RPMI-10 medium containing 300 U/mL hGM-CSF and 200 U/mL hIL-4 for 4 days.
Antibodies and flow cytometry
FITC-conjugated anti-CD3, CD4, CD44, CD69 and CD122; PE-conjugated anti–IL-17A and NK1.1; PerCP-conjugated anti-CD8α; biotin-conjugated anti-CD80, CD86, CD11b, CD45.1, and CD45.2; and APC-conjugated anti-TCRβ, CD11c, and IFN-γ were purchased from BD Bioscience or eBioscience. Biotin-conjugated anti–human CD1b were purchased from Ancell. For cell surface staining, cells were incubated with 2.4G2 blocking mAb for 15 minutes, and then stained with the appropriate combinations of mAb. For intracellular staining, cells were fixed in 4% paraformaldehyde for 5 minutes at room temperature, permeabilized in PBS containing 1% BSA and 0.1% saponin for 10 minutes, followed by intracellular staining with specific mAb for 30 minutes. PLZF expression on thymocytes was analyzed via intracellular staining using the FoxP3 staining buffer set (eBioscience) with 4 μg/mL anti-PLZF mAb (Santa Cruz Biotechnology). Stained cells were collected and analyzed using a FACSCanto II with FlowJo Version 8.8.6 software (TreeStar).
Cytokine release assay
Purified HJ1 T cells (1-2 × 105) were stimulated with plate-bound anti-CD3 (5 μg/mL) or cultured together with TLR agonist-treated, LM-infected murine BMDC or human monocyte-derived DC (1 × 105 cells) in RPMI-10 medium. After 48 hours, cytokine production was measured using a mouse Th1/Th2/Th17 Cytometric Bead Array kit or by ELISA. For treatment with TLR agonists, DC were stimulated overnight with Pam3Cys (100 ng/mL) or LPS (100 ng/mL). To infect BMDC with LM, BMDC were infected with LM at a MOI of 2 for 1 hour. Infected BMDC were washed and cultured in medium containing gentamicin (10 μg/mL) for 1 hour before coculture with T cells. For antibody blocking experiments, 10 μg/mL of anti-CD1b, -CD1c, –IL-12 p40, or –IL-18 were added to the coculture. For the detection of intracellular cytokine production by HJ1 T cells, 3 × 105 liver leukocytes from HJ1Tg/hCD1Tg/Rag−/− mice were stimulated with anti-CD3 (10μg/mL)/CD28 (2μg/mL) for 6 hours. Brefeldin A was added 2 hours before the end of culture.
CTL assay
CTL effectors were established by stimulating splenocytes with 1 μg/mL Concanavalin A (ConA) for 3 days in RPMI-10, followed by culture in supplemented Mischell Dutton medium with IL-2 (20 U/mL) for 4 days. Target cells were labeled with 50 μCi [51Cr] sodium chromate for 1 hour and cultured with HJ1Tg ConA blasts for 4 hours at 37°C. The percentage of specific lysis was calculated as [(experimental release − spontaneous release) / (maximum release − spontaneous release)] × 100.
Generation of BM chimeras
BM cells were depleted of mature T cells with anti-Thy1.2 mAb and rabbit complement. T cell–depleted BM cells (1 × 107) were injected intravenously per irradiated recipient. Lymphocytes isolated from recipient mice were analyzed by flow cytometry 6-7 weeks after transfer.
Adoptive transfer and listerial infection
Enriched HJ1 T cells (1-2 × 107) were adoptively transferred into recipient mice. One hour after cell transfer, recipient mice were challenged intravenously with 1 × 104 CFU (for hCD1Tg mice) or 2 × 103 CFU (for hCD1Tg/IFN-γ−/− mice) of EGD strain of Listeria monocytogenes. Three days after infection, bacterial burden of infected recipients were determined as described previously.28
Statistical analysis
Statistical analyses were performed using Prism Version 4b software (GraphPad). The statistical significance of differences between experimental groups was analyzed using a Student t test. A P value of < .05 was considered statistically significant.
Results
Generation of an autoreactive CD1b-restricted TCR transgenic mouse model
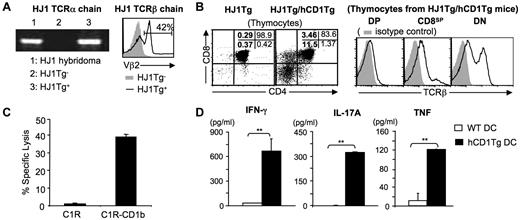
Most studies of group 1 CD1-restricted T cells have focused on their role in antigen-specific anti-mycobacterial host defense. However, a large proportion of group 1 CD1-restricted T-cell lines isolated thus far are autoreactive,2,4,14,20-23 raising the possibility that this unique T-cell subset plays a significant role in immunity. To examine the function of autoreactive group 1 CD1-restricted T cells in vivo, we generated HJ1Tg mice that express a TCR derived from the CD1b-autoreactive T-cell clone HJ1.14 Rearranged variable region fragments from HJ1 CTL (Vα8.5-Jα14 and Vβ2-Dβ2-Jβ2.3) were cloned into TCR cassette vectors containing natural promoter and enhancer elements to direct expression of rearranged TCR genes in HJ1Tg mice. As no anti-Vα8.5 antibody is commercially available, HJ1Tg founders and their progeny were screened for the presence of Vα8.5-Jα14 gene fragment by PCR and for the surface expression of Vβ2 by flow cytometry (Figure 1A). Two founders were identified, and the founder with the higher frequency of Vβ2+ T cells was crossed onto the hCD1Tg background (HJ1Tg/hCD1Tg). To eliminate the interference of endogenous TCR in the analysis of HJ1 T cells, we further crossed HJ1Tg/hCD1Tg mice onto a Rag−/− background. All HJ1Tg mice used in this study are on a Rag−/− background.
HJ1 T cells are autoreactive and restricted to CD1b. (A) Generation of HJ1Tg mice. The presence of rearranged Vα8.5-Jα14 TCRα chain was examined by PCR from genomic DNA of HJ1Tg+, HJ1Tg− littermates, and HJ1 T-cell hybridoma (left). Histograms depict FACS staining for Vβ2 of blood lymphocytes isolated from HJ1Tg+ (open histogram) and HJ1Tg− littermates (filled histogram; right). Results are representative of 4 individual experiments. (B) CD1b is required for the development of HJ1 thymocytes. Thymocytes were isolated from HJ1Tg/Rag−/− and HJ1Tg/hCD1Tg/Rag−/− mice, stained with mAb against CD4, CD8, and TCRβ, and analyzed by flow cytometry. Dot plots depict CD4 and CD8 staining; numbers indicate cell percentages in each quadrant (left). Histograms represent TCRβ expression (open histogram) on thymocytes isolated from HJ1Tg/hCD1Tg/Rag−/− mice in DP, CD8SP and DN gates (right). Isotype control staining is depicted as filled histograms. Results are representative of 3 independent experiments. (C) HJ1 T cells are restricted to CD1b. ConA-blasts generated from the splenocytes of HJ1Tg/hCD1Tg/Rag−/− mice were tested for the cytotoxic activity against C1R and CD1b-transfected C1R at an E/T ratio of 30:1. Results are representative of 2 experiments. (D) HJ1 T cells are autoreactive. HJ1 T cells were enriched from the liver leukocytes of HJ1Tg/hCD1Tg/Rag−/− mice and were cultured with WT or hCD1Tg BMDC. Cytokines in the supernatant were measured by Th1/Th2/Th17 CBA kit. Results are representative of 3 experiments.
HJ1 T cells are autoreactive and restricted to CD1b. (A) Generation of HJ1Tg mice. The presence of rearranged Vα8.5-Jα14 TCRα chain was examined by PCR from genomic DNA of HJ1Tg+, HJ1Tg− littermates, and HJ1 T-cell hybridoma (left). Histograms depict FACS staining for Vβ2 of blood lymphocytes isolated from HJ1Tg+ (open histogram) and HJ1Tg− littermates (filled histogram; right). Results are representative of 4 individual experiments. (B) CD1b is required for the development of HJ1 thymocytes. Thymocytes were isolated from HJ1Tg/Rag−/− and HJ1Tg/hCD1Tg/Rag−/− mice, stained with mAb against CD4, CD8, and TCRβ, and analyzed by flow cytometry. Dot plots depict CD4 and CD8 staining; numbers indicate cell percentages in each quadrant (left). Histograms represent TCRβ expression (open histogram) on thymocytes isolated from HJ1Tg/hCD1Tg/Rag−/− mice in DP, CD8SP and DN gates (right). Isotype control staining is depicted as filled histograms. Results are representative of 3 independent experiments. (C) HJ1 T cells are restricted to CD1b. ConA-blasts generated from the splenocytes of HJ1Tg/hCD1Tg/Rag−/− mice were tested for the cytotoxic activity against C1R and CD1b-transfected C1R at an E/T ratio of 30:1. Results are representative of 2 experiments. (D) HJ1 T cells are autoreactive. HJ1 T cells were enriched from the liver leukocytes of HJ1Tg/hCD1Tg/Rag−/− mice and were cultured with WT or hCD1Tg BMDC. Cytokines in the supernatant were measured by Th1/Th2/Th17 CBA kit. Results are representative of 3 experiments.
Flow cytometric analysis of thymocytes in HJ1Tg and HJ1Tg/hCD1Tg mice revealed that while most of the thymocytes in HJ1Tg mice were arrested at the DP stage, a significant proportion of thymocytes in HJ1Tg/hCD1Tg mice were DN or CD8SP (Figure 1B). In addition, most DN and CD8SP thymocytes found in HJ1Tg/hCD1Tg mice have up-regulated TCR expression, indicating that they have undergone positive selection (Figure 1B). These data suggest that CD1b molecules are required for the positive selection of HJ1 thymocytes. To assess the function of the HJ1 TCR transgenes, we stimulated splenocytes from HJ1Tg/hCD1Tg mice with ConA. Similar to HJ1 CTL, ConA-activated HJ1 T cells were capable of lysing CD1b-transfected C1R cells, but failed to kill untransfected C1R cells (Figure 1C). In addition, HJ1 T cells produce the proinflammatory cytokines IFN-γ, IL-17A, and TNF in response to stimulation with hCD1Tg BMDC, but not WT BMDC (Figure 1D). Taken together, these data show that the HJ1 T cells isolated from HJ1Tg/hCD1Tg mice have similar properties to the original HJ1 CTL and suggest that HJ1Tg/hCD1Tg mice are a viable model for studying the development and function of CD1b-autoreactive T cells.
HJ1 T cells in HJ1Tg/hCD1Tg mice exhibit an NKT cell-like phenotype
To further characterize HJ1 T cells, we examined the surface phenotype of HJ1 T cells isolated from HJ1Tg/hCD1Tg mice by flow cytometry. Interestingly, a significant proportion of HJ1 T cells in HJ1Tg/hCD1Tg mice expressed NK1.1 (Figure 2A). These NK1.1+ HJ1 T cells were highly enriched in the liver, reminiscent of CD1d-restricted iNKT cells. Although CD1b is required for efficient positive selection of HJ1 T cells in the thymus (Figure 1B), some HJ1 T cells are detected in the periphery of HJ1Tg mice (CD1b-negative), suggesting that other MHC molecules may contribute to the development of HJ1 T cells in the absence of CD1b. Notably, NK1.1+ HJ1 T cells were virtually absent in HJ1Tg mice, suggesting that the selection/ development of this unique T-cell population is CD1b-dependent (Figure 2A).
HJ1 T cells found in HJ1Tg/hCD1Tg mice exhibit an activated phenotype. Cells were isolated from the thymus, spleen, and liver of HJ1Tg/Rag−/− and HJ1Tg/hCD1Tg/Rag−/− mice, stained with mAb against various cell surface markers, and analyzed by flow cytometry. Results are representative of 3 independent experiments. (A) NK1.1+ HJ1 T cells require CD1b molecules for proper development. Cells were stained with mAb against CD3 and NK1.1. The percentages of CD3+NK1.1+ and CD3+NK1.1− T cells in each organ are indicated. (B) The majority of the HJ1 T cells developed in the presence of CD1b molecules are DN. Liver leukocytes were stained with mAb against CD3, NK1.1, CD4 and CD8α. Dot plots depict the co-receptor usage of HJ1 T cells in the CD3+NK1.1+ and CD3+NK1.1− gates. The percentage of cells in each quadrant is indicated. (C) HJ1 T cells isolated from HJ1Tg/hCD1Tg/Rag−/− mice express activation markers. Cells from HJ1Tg/hCD1Tg/Rag−/− and Vα14Tg mice were stained with CD3 together with CD44, CD69, or CD122. Histograms depict the surface expression of CD44, CD69 and CD122 on the CD3+ cells. Isotype control staining is depicted as filled histograms and the staining of Vα14Tg T cells and HJ1 T cells is depicted as dotted line and solid line, respectively. (D) HJ1 thymocytes express the transcription factor PLZF. Thymocytes from HJ1Tg/hCD1Tg/Rag−/− mice were surface stained with CD3 and intracellularly stained for PLZF. The specificity of the anti-PLZF antibody was confirmed by staining conventional T cells (TCRβ+CD1d/α-GalCer tetramer− gate) and iNKT cells (TCRβ+CD1d/α-GalCer tetramer+ gate) isolated from WT mice.
HJ1 T cells found in HJ1Tg/hCD1Tg mice exhibit an activated phenotype. Cells were isolated from the thymus, spleen, and liver of HJ1Tg/Rag−/− and HJ1Tg/hCD1Tg/Rag−/− mice, stained with mAb against various cell surface markers, and analyzed by flow cytometry. Results are representative of 3 independent experiments. (A) NK1.1+ HJ1 T cells require CD1b molecules for proper development. Cells were stained with mAb against CD3 and NK1.1. The percentages of CD3+NK1.1+ and CD3+NK1.1− T cells in each organ are indicated. (B) The majority of the HJ1 T cells developed in the presence of CD1b molecules are DN. Liver leukocytes were stained with mAb against CD3, NK1.1, CD4 and CD8α. Dot plots depict the co-receptor usage of HJ1 T cells in the CD3+NK1.1+ and CD3+NK1.1− gates. The percentage of cells in each quadrant is indicated. (C) HJ1 T cells isolated from HJ1Tg/hCD1Tg/Rag−/− mice express activation markers. Cells from HJ1Tg/hCD1Tg/Rag−/− and Vα14Tg mice were stained with CD3 together with CD44, CD69, or CD122. Histograms depict the surface expression of CD44, CD69 and CD122 on the CD3+ cells. Isotype control staining is depicted as filled histograms and the staining of Vα14Tg T cells and HJ1 T cells is depicted as dotted line and solid line, respectively. (D) HJ1 thymocytes express the transcription factor PLZF. Thymocytes from HJ1Tg/hCD1Tg/Rag−/− mice were surface stained with CD3 and intracellularly stained for PLZF. The specificity of the anti-PLZF antibody was confirmed by staining conventional T cells (TCRβ+CD1d/α-GalCer tetramer− gate) and iNKT cells (TCRβ+CD1d/α-GalCer tetramer+ gate) isolated from WT mice.
Analysis of the coreceptor usage of mature HJ1 T cells that develop in HJ1Tg/hCD1Tg mice showed that both NK1.1+ and NK1.1− HJ1 T cells were predominantly DN, with a small population of cells expressing CD8, similar to the original HJ1 CTL (Figure 2B). In addition, like iNKT cells, HJ1 T cells found in HJ1Tg/hCD1Tg mice exhibited an activated phenotype (CD44+ CD69+CD122+) in the thymus, spleen and liver (Figure 2C), suggesting that autoreactive HJ1 T cells acquire this unique phenotype during thymic development before they exit to the periphery. To further define the stage that HJ1 T cells acquire the “NKT cell-like” phenotype, we examined the expression of NK1.1, CD122 and CD44 on various thymic populations, including DP (mainly pre-selection), DPdull (immediately following positive selection), and DN (mostly mature) thymocytes. A substantial proportion of DPdull and DN thymocytes in HJ1Tg/hCD1Tg mice, but not in HJ1Tg mice, expresses NK1.1, CD44 and CD122 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This finding further suggests that interaction between HJ1 TCR and CD1b during thymic selection contributes to the unique surface phenotype of HJ1 T cells.
The transcription factor PLZF has been recently shown to be expressed by several “nonconventional” T-cell subsets that display an activated phenotype, including iNKT cells, MR1-restricted Vα19 T cells, and some γδ T cells.29-33 We found that HJ1 thymocytes from HJ1Tg/hCD1Tg mice expressed similar levels of PLZF to iNKT cells (Figure 2D), indicating that PLZF might play a role in HJ1 T-cell development. Taken together, our data show that CD1b-autoreactive HJ1 T cells that develop in the presence of CD1b molecules share a similar phenotype with iNKT cells and may have similar developmental requirements.
NK1.1+ HJ1 T cells are positively selected by group 1 CD1- expressing hematopoietic cells
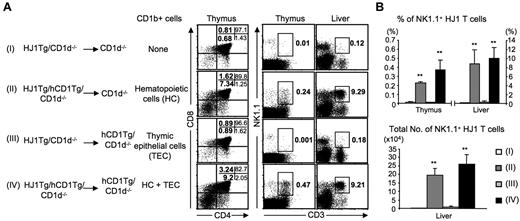
Positive selection of iNKT cells is mediated by thymocytes, as opposed to the thymic epithelial cells that mediate the selection of conventional T cells.10 Given the similar phenotype between iNKT cells and the HJ1 T cells that develop in HJ1Tg/hCD1Tg mice, we hypothesized that NK1.1+ HJ1 T cells might be selected by CD1b-expressing hematopoietic cells. To test this hypothesis, we established chimeric mice by adoptive transfer of bone marrow cells from HJ1Tg and HJ1Tg/hCD1Tg mice on a CD1d−/− background into irradiated CD1d−/− and hCD1Tg/CD1d−/− recipients. Mice on a CD1d−/− background were used to generate chimeric mice to eliminate the possibility that CD1d might contribute to the development/selection of HJ1 T cells. Six to 7 weeks after adoptive transfer, T cells from recipient mice were examined by flow cytometric analysis.
We found that the development of NK1.1+ HJ1 T cells was severely impaired in recipients reconstituted with HJ1Tg/CD1d−/− bone marrow, as most of the thymocytes in these mice were arrested at the DP stage (Figure 3A, Groups I and III). In addition, both the proportion and total numbers of NK1.1+ HJ1 T cells were significantly decreased compared with the positive control group (HJ1Tg/hCD1Tg/CD1d−/− → hCD1Tg/CD1d−/−, Group IV; Figure 3A-B), confirming the requirement of CD1b in the development of NK1.1+ HJ1 T cells. In contrast, the proportion and total numbers of NK1.1+ HJ1 T cells detected in CD1d−/−/Rag−/− mice that received HJ1Tg/hCD1Tg/CD1d−/− bone marrow (Group II) were comparable to those of the positive control group (Group IV), indicating that CD1b-expressing hematopoietic cells are necessary and sufficient to mediate the positive selection of NK1.1+ HJ1 T cells. In addition, a significantly higher proportion of NK1.1− HJ1 T cells were present in the periphery of hCD1Tg/CD1d−/− recipients reconstituted with HJ1Tg/CD1d−/− bone marrow (Group III), compared with those detected in CD1d−/− recipients (Group I, Figure 3A), suggesting a potential contribution of CD1b expression on nonhematopoietic cells to the survival and expansion of NK1.1− HJ1 T cells that might be selected by other MHC molecules in the thymus. Although not all the HJ1 T cells selected by CD1b-expressing hematopoietic cells are NK1.1+, our data clearly demonstrates that NK1.1+ HJ1 T cells, like iNKT cells, are positively selected by CD1b-expressing hematopoietic cells.
Group 1 CD1-expressing hematopoietic cells mediate the positive selection of NK1.1+ HJ1 T cells. CD1d−/− or hCD1Tg/CD1d−/− mice were reconstituted with BM cells from HJ1Tg/CD1d−/− or HJ1Tg/hCD1Tg/CD1d−/− mice (both donor and recipient mice are on a Rag−/− background). Six to 7 weeks later, cells were isolated from each group, stained with mAb against CD4, CD8, CD3 and NK1.1, and analyzed by flow cytometry. (A) Dot plots depict the proportion of CD4- and CD8-expressing thymocytes in each quadrant, as well as the proportion of NK1.1-expressing T cells in the lymphocyte gate. Data are representative of 3 independent experiments with 2 mice in each group. (B) Bar graphs depict the mean ± SD for the percentages (top panel) and absolute numbers (bottom panel) of NK1.1+CD3+ T cells in the liver lymphocyte gate. Statistical significance was evaluated by comparing Groups II, III, and IV with the negative control group (Group I). **P < .01, n = 6 for each experimental group.
Group 1 CD1-expressing hematopoietic cells mediate the positive selection of NK1.1+ HJ1 T cells. CD1d−/− or hCD1Tg/CD1d−/− mice were reconstituted with BM cells from HJ1Tg/CD1d−/− or HJ1Tg/hCD1Tg/CD1d−/− mice (both donor and recipient mice are on a Rag−/− background). Six to 7 weeks later, cells were isolated from each group, stained with mAb against CD4, CD8, CD3 and NK1.1, and analyzed by flow cytometry. (A) Dot plots depict the proportion of CD4- and CD8-expressing thymocytes in each quadrant, as well as the proportion of NK1.1-expressing T cells in the lymphocyte gate. Data are representative of 3 independent experiments with 2 mice in each group. (B) Bar graphs depict the mean ± SD for the percentages (top panel) and absolute numbers (bottom panel) of NK1.1+CD3+ T cells in the liver lymphocyte gate. Statistical significance was evaluated by comparing Groups II, III, and IV with the negative control group (Group I). **P < .01, n = 6 for each experimental group.
Bacterial infection and TLR stimulation enhance the autoreactivity of HJ1 T cells
Recent studies have shown that the presence of bacteria or bacterial products can enhance the activation of CD1-autoreactive T cells.2,5,24,34,35 We therefore examined the effect of TLR agonists and Listeria monocytogenes (LM) infection on HJ1 T-cell activation. The TLR agonists Pam3Cys(TLR2) and LPS (TLR4) induced substantial BMDC maturation, as assessed by the up-regulation of the costimulatory molecules CD80 and CD86 (supplemental Figure 2). Treatment of hCD1Tg BMDC with these TLR agonists led to increased IFN-γ and IL-17A production by HJ1 T cells (Figure 4A). This enhanced cytokine production required the interaction of TCR and CD1b, as the addition of an anti-CD1b mAb, but not an irrelevant antibody, resulted in a 60% reduction in IL-17A production by HJ1 T cells (supplemental Figure 3). No detectable levels of IL-17A and IFN-γ were found in cultures consisting of HJ1 T cells or BMDC alone, even after the addition of TLR agonists (data not shown). A similar pattern of reactivity was observed when HJ1 T cells were stimulated with human monocyte-derived DC, suggesting that the endogenous lipid antigen(s) recognized by HJ1 T cells are conserved between mice and humans (Figure 4B).
Infection of DC with listeria or pretreatment of DC with TLR agonists enhances cytokine secretion by HJ1 T cells. HJ1 T cells were enriched from the liver of HJ1Tg/hCD1Tg/Rag−/− mice. (A) WT and hCD1Tg BMDC were either left untreated, were pretreated with Pam3Cys or LPS, or were infected with LM before co-culture with HJ1 T cells. After 48 hours, cytokine levels in the supernatant were detected by ELISA. Results are expressed as the mean ± SD. Statistical significance was evaluated by comparing cytokine secretion between HJ1 T-cell cultures stimulated with treated hCD1Tg BMDC and cultures stimulated with untreated hCD1Tg BMDC. (B) HJ1 T cells were cocultured with human monocyte-derived DC in the presence of anti-CD1b or isotype mAb for 48 hours. Cytokine secretions were examined by ELISA. Results are expressed as the mean ± SD. Statistical significance was evaluated by comparing cytokine secretion by HJ1 T-cell cultures in the presence of anti-CD1b with in the presence of isotype Ab. Data are representative of 2 independent experiments. (C) HJ1Tg T cells and Vα14Tg NKT cells were stimulated with Pam3Cys-pulsed WT or hCD1Tg BMDC for 48 hours. Bar graphs depict the mean ± SD for cytokine levels in the supernatant as detected by ELISA. Statistical significance was evaluated by comparing cytokine secretion between HJ1 T-cell cultures stimulated with treated-hCD1Tg BMDC and cultures stimulated with treated-WT BMDC, or by comparing cytokine secretion between HJ1Tg T cells and iNKT cells on stimulation with Pam3cys-treated hCD1Tg BMDC. **P < .01. Data are representative of 3 independent experiments.
Infection of DC with listeria or pretreatment of DC with TLR agonists enhances cytokine secretion by HJ1 T cells. HJ1 T cells were enriched from the liver of HJ1Tg/hCD1Tg/Rag−/− mice. (A) WT and hCD1Tg BMDC were either left untreated, were pretreated with Pam3Cys or LPS, or were infected with LM before co-culture with HJ1 T cells. After 48 hours, cytokine levels in the supernatant were detected by ELISA. Results are expressed as the mean ± SD. Statistical significance was evaluated by comparing cytokine secretion between HJ1 T-cell cultures stimulated with treated hCD1Tg BMDC and cultures stimulated with untreated hCD1Tg BMDC. (B) HJ1 T cells were cocultured with human monocyte-derived DC in the presence of anti-CD1b or isotype mAb for 48 hours. Cytokine secretions were examined by ELISA. Results are expressed as the mean ± SD. Statistical significance was evaluated by comparing cytokine secretion by HJ1 T-cell cultures in the presence of anti-CD1b with in the presence of isotype Ab. Data are representative of 2 independent experiments. (C) HJ1Tg T cells and Vα14Tg NKT cells were stimulated with Pam3Cys-pulsed WT or hCD1Tg BMDC for 48 hours. Bar graphs depict the mean ± SD for cytokine levels in the supernatant as detected by ELISA. Statistical significance was evaluated by comparing cytokine secretion between HJ1 T-cell cultures stimulated with treated-hCD1Tg BMDC and cultures stimulated with treated-WT BMDC, or by comparing cytokine secretion between HJ1Tg T cells and iNKT cells on stimulation with Pam3cys-treated hCD1Tg BMDC. **P < .01. Data are representative of 3 independent experiments.
To examine whether bacterial infection affects the capacity of DC to stimulate HJ1 T cells, we used LM-infected DC as stimulators. LM-infected hCD1Tg BMDC enhanced the secretion of both IFN-γ and IL-17A by HJ1 T cells (Figure 4A). It is noteworthy that HJ1 T cells produced substantial amounts of IFN-γ and IL-17A in response to LM-infected WT BMDC, albeit these levels were significantly lower than those induced by LM-infected hCD1Tg BMDC. It is possible that the high levels of pro-inflammatory cytokines produced by LM-infected DC lead to HJ1 T-cell activation in a TCR-independent manner. Nevertheless, our data indicate that, in addition to TCR engagement, signals derived from TLR-mediated DC activation play a role in regulating the autoreactivity of HJ1 T cells.
To compare the amount of cytokines produced by HJ1 T cells relative to iNKT cells, we examined cytokine secretion by HJ1 T cells and Vα14Tg NKT cells in response to Pam3Cys-pulsed BMDC. Both Pam3Cys-treated WT and hCD1Tg BMDC stimulated Vα14Tg NKT cells to produce Th1 (IFN-γ) and Th17 (IL-17A and IL-22) cytokines to a similar capacity, indicating that the presence of group 1 CD1 molecules does not affect the reactivity of iNKT cells (Figure 4C). Compared with Vα14Tg NKT cells, HJ1 T cells produced slightly lower levels of IFN-γ but comparable amounts of IL-17A in response to Pam3Cys-pulsed hCD1Tg BMDC. Consistently, more IL-22 was detected in co-cultures of HJ1 T cells and Pam3Cys-pulsed BMDC than in co-cultures containing Vα14Tg NKT cells. The distinction between the cytokine production profiles of these two innate T-cell types suggests that they may play differential roles in the regulation of immune responses.
DC-derived cytokines augments HJ1 T-cell activation
DC-derived cytokines IL-12 and IL-18 have been shown to enhance IFN-γ secretion by iNKT cells.5,35,36 To investigate whether similar mechanism(s) contribute to the enhanced HJ1 T-cell activation in response to Pam3Cys-treated hCD1Tg BMDC, we examined the effect of anti–IL-12p40 and anti–IL-18 on HJ1 T-cell activation. In a co-culture of HJ1 T cells and Pam3Cys-pulsed hCD1Tg BMDC, the addition of anti–IL-12p40 reduced IFN-γ secretion by > 80%, while the addition of an isotype control mAb had no effect (Figure 5). Similar results were found when anti–IL-12p40 was added to a co-culture of HJ1 T cells and LM-infected hCD1Tg BMDC (supplemental Figure 4). Because the IL-12p40 chain is common to both IL-12 and IL-23, the presence of anti–IL-12p40 understandably reduced IL-17A secretion by HJ1 T cells on stimulation with either Pam3Cys-pulsed or LM-infected hCD1Tg BMDC. In contrast, the addition of anti–IL-18 resulted in only a slight decrease in IFN-γ secretion by HJ1 T cells in response to LM-infected hCD1Tg BMDC, and had no effect on stimulation with Pam3Cys-pulsed hCD1Tg BMDC. Therefore, our data indicate that the IL-12/IL-23, but not IL-18, produced by TLR ligand-activated DC plays an important role in the enhancement of cytokine secretion by CD1b-autoreactive HJ1 T cells.
DC-derived cytokines enhance the activation of HJ1 T cells on stimulation with Pam3Cys-treated hCD1Tg BMDC. HJ1 T cells were enriched from liver leukocytes isolated from HJ1Tg/hCD1Tg/Rag−/− mice, and were co-cultured with Pam3Cys-treated hCD1Tg BMDC. Anti–IL-12 mAb, anti–IL-18 mAb, both anti–IL-12 and anti–IL-18 together, or an isotype-matched control mAb were added to co-cultures. Bar graphs depict the mean ± SD for the amount of IFN-γ or IL-17A in culture supernatants. Statistical significance was evaluated by comparing each experimental group with the isotype control group. *P < .05; **P < .01. Data are representative of 3 independent assays.
DC-derived cytokines enhance the activation of HJ1 T cells on stimulation with Pam3Cys-treated hCD1Tg BMDC. HJ1 T cells were enriched from liver leukocytes isolated from HJ1Tg/hCD1Tg/Rag−/− mice, and were co-cultured with Pam3Cys-treated hCD1Tg BMDC. Anti–IL-12 mAb, anti–IL-18 mAb, both anti–IL-12 and anti–IL-18 together, or an isotype-matched control mAb were added to co-cultures. Bar graphs depict the mean ± SD for the amount of IFN-γ or IL-17A in culture supernatants. Statistical significance was evaluated by comparing each experimental group with the isotype control group. *P < .05; **P < .01. Data are representative of 3 independent assays.
HJ1 T cells are protective against listerial infection
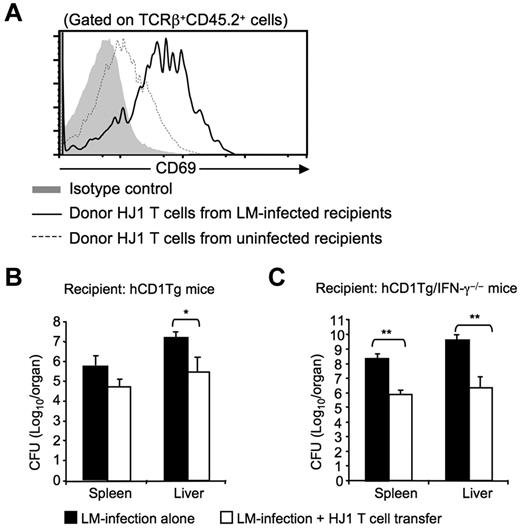
Our in vitro data show that HJ1 T cells produce substantial amounts of IFN-γ and IL-17A in response to LM-infected hCD1Tg BMDC, raising the possibility that HJ1 T cells may contribute to immunity against LM infection. To address this issue, we adoptively transferred HJ1 T cells (CD45.2+) into CD45.1 congenic hCD1Tg mice, and subsequently challenged the recipients with virulent LM strain. hCD1Tg mice receiving LM infection alone or transferred HJ1 T cells alone were used as controls. Three days after infection, the activation status of donor HJ1 T cells and the bacterial burden in recipient mice were examined. We found that the mean fluorescence intensity of the early activation marker CD69 on HJ1 T cells was increased in the infected recipient mice compared with those isolated from uninfected mice (Figure 6A), suggesting that HJ1 T cells are activated during LM infection. In addition, hCD1Tg mice that received HJ1 T cells displayed a > 10-fold reduction in bacterial burden in the liver (Figure 6B) compared with the liver of infected-hCD1Tg mice that had not received HJ1 T cells, demonstrating that HJ1 T cells contribute to anti-listerial immunity. The anti-listerial effect of HJ1 T cells was more profound in hCD1Tg/IFN-γ−/− recipient mice, in which a transfer of HJ1 T cells decreased bacterial burden by > 100-fold in both the spleen and liver (Figure 6B). Taken together, our data suggest that CD1b-autoreactive HJ1 T cells can be activated in vivo during infection and contribute to early host defense against listerial infection.
HJ1 T cells protect against LM infection. (A) Adoptively transferred HJ1 T cells are activated on LM infection. hCD1Tg congenic mice (CD45.1+) were adoptively transferred with 1-2 × 107 HJ1 T cells (CD45.2+). One hour after transfer, recipient mice were either left alone or intravenously infected with LM. Three days after infection, mice were killed. Single cell suspensions were isolated from the liver of LM-infected or uninfected hCD1Tg congenic mice, stained with mAb against TCRβ, CD69 and CD45.2, and analyzed by flow cytometry. Histograms depict CD69 expression on donor HJ1 T cells based on TCRβ+CD45.2+ gating (gray: isotype control; dotted line: donor HJ1 T cells from uninfected recipient mice; solid line: donor HJ1 T cells from infected recipient mice). Results are representative of 3 independent experiments. (B-C) Adoptive transfer of HJ1 T cells reduces bacterial burden in hCD1Tg (B) and hCD1Tg/IFNγ−/− mice (C). hCD1Tg and hCD1Tg/IFN-γ−/− recipient mice were adoptively transferred with 1-2 × 107 HJ1 T cells. One hour after cell transfer, recipient mice were infected with LM. Three days after infection, spleen and liver were harvested and the bacterial burden was determined. Bar graphs depict the mean ± SD for the bacterial burden in the spleen and liver. Statistical significance was evaluated through comparison of infected mice that had received a transfer of HJ1 T cells and infected recipients that did not receive a transfer. *P < .05; **P < .01. Data were pooled from 4 independent experiments (n = 4 for each group).
HJ1 T cells protect against LM infection. (A) Adoptively transferred HJ1 T cells are activated on LM infection. hCD1Tg congenic mice (CD45.1+) were adoptively transferred with 1-2 × 107 HJ1 T cells (CD45.2+). One hour after transfer, recipient mice were either left alone or intravenously infected with LM. Three days after infection, mice were killed. Single cell suspensions were isolated from the liver of LM-infected or uninfected hCD1Tg congenic mice, stained with mAb against TCRβ, CD69 and CD45.2, and analyzed by flow cytometry. Histograms depict CD69 expression on donor HJ1 T cells based on TCRβ+CD45.2+ gating (gray: isotype control; dotted line: donor HJ1 T cells from uninfected recipient mice; solid line: donor HJ1 T cells from infected recipient mice). Results are representative of 3 independent experiments. (B-C) Adoptive transfer of HJ1 T cells reduces bacterial burden in hCD1Tg (B) and hCD1Tg/IFNγ−/− mice (C). hCD1Tg and hCD1Tg/IFN-γ−/− recipient mice were adoptively transferred with 1-2 × 107 HJ1 T cells. One hour after cell transfer, recipient mice were infected with LM. Three days after infection, spleen and liver were harvested and the bacterial burden was determined. Bar graphs depict the mean ± SD for the bacterial burden in the spleen and liver. Statistical significance was evaluated through comparison of infected mice that had received a transfer of HJ1 T cells and infected recipients that did not receive a transfer. *P < .05; **P < .01. Data were pooled from 4 independent experiments (n = 4 for each group).
Discussion
In this study, we examine the developmental requirements and in vivo function of CD1b-autoreactive T cells in HJ1Tg/hCD1Tg mice. Our results demonstrate that the CD1b-autoreactive HJ1 T cells isolated from HJ1Tg/hCD1Tg mice share several features with iNKT cells. First, HJ1 T cells exhibit an activated phenotype and express PLZF, the transcription factor involved in the development of innate-like T cells.29-33 Second, a subset of HJ1 T cells that expresses NK1.1 is selected by CD1b-expressing hematopoietic cells and is highly enriched in the liver. Third, HJ1 T cells can be activated by CD1b-expressing DC; the presence of bacteria (LM) or TLR agonists (Pam3Cys and LPS) enhances this autoreactivity by stimulating DC to produce IL-12/IL-23. These similarities suggest that HJ1 T cells may function similarly to iNKT cells, bridging innate and adaptive immune responses.
Like HJ1 T cells, a large proportion of human group 1 CD1-restricted T cells isolated thus far recognize group 1 CD1-expressing cells in the absence of exogenous lipid antigen, raising the question as to how these autoreactive T cells escape negative selection in the thymus. We found that the total number of thymocytes is severely reduced in HJ1Tg/hCD1Tg mice (5.8 ± 1.4 × 106) compared with that in HJ1Tg mice (1.21 ± 0.3 × 108), suggesting that HJ1 thymocytes do undergo negative selection on the engagement with CD1b-expressing cells. Similar to humans, CD1b is expressed at modest levels on cortical thymocytes and at high levels on DC in hCD1Tg mice. Therefore, it is likely that the negative selection of HJ1 T cells is mediated by CD1b-expressing DC in the thymus. However, this negative selection is incomplete as a sizable HJ1 T-cell population is found in the periphery of HJ1Tg/hCD1Tg mice. Notably, the HJ1 T cells that develop in the hCD1Tg background express lower levels of surface TCR compared with HJ1 T cells that develop in a WT background (data not shown). Furthermore, a substantial proportion of HJ1 T cells in HJ1Tg/hCD1Tg mice do not express detectable amounts of CD3 and TCR on the cell surface, yet express significant levels of intracellular TCR (supplemental Figure 5). This observation suggests that down-regulation of surface TCR expression might be one of the mechanisms by which autoreactive group 1 CD1-restricted T cells avoid both negative selection in the thymus and overt autoreactivity in the periphery.
We demonstrated that the development of NK1.1+ HJ1 T cells is dependent on positive selection by CD1b-expressing hematopoietic cells. The hematopoietic cell type that mediates the selection of NK1.1+ HJ1 T cells is likely to be cortical thymocytes, as CD1b is not expressed on mature thymocytes. As for iNKT cells, this unique developmental pathway may contribute to the distinct phenotype (expression of NK and activation markers) associated with HJ1 T cells in naive HJ1Tg/hCD1Tg mice. This notion is supported by our finding that HJ1 T cells found in HJ Tg mice do not exhibit activated phenotype (supplemental Figure 6). In addition, we have performed intracellular cytokine staining to examine whether NK1.1+ and NK1.1− populations of HJ1 T cells that developed in HJ1Tg/hCD1Tg mice have differential cytokine producing capacity. On stimulation with anti-CD3/CD28, a significant higher proportion of NK1.1+ HJ1 T cells produces IFN-γ compared with NK1.1− HJ1 T cells, while the proportion of IL-17A–producing cells were comparable between these 2 subsets (supplemental Figure 7).
It is worth mentioning that most of the group 1 CD1-restricted T-cell lines isolated from humans and hCD1Tg mice (including HJ1) lack expression of NK markers,14,17,24 perhaps resulting from repeated in vitro stimulation or in vivo activation. Loss of NK1.1 expression on murine iNKT cells on activation is already well-described.37-39 Thus, our finding suggests that autoreactive group 1 CD1-restricted T cells may contribute to the NKT-cell repertoire in humans. It has been reported that T cells expressing NK receptors including CD56, CD161 and CD94 are substantially enriched in adult human liver.40,41 In fact, one-third of human hepatic T cells have been shown to express NK markers. Among them, only a small proportion expresses invariant TCR chains.40,41 Although some of these NKT cells could be CD1d-restricted type II NKT cells42,43 or activated classic MHC-restricted T cells,44 it is possible that autoreactive group 1 CD1-restricted T cells may also reside within the NKT-cell compartment of the human liver. Our finding that NK1.1+ HJ1 T cells are highly enriched in the liver of HJ1Tg/hCD1Tg mice supports this notion.
Unlike iNKT cells, HJ1Tg T cells produce both IFN-γ and IL-17A, but not IL-4, in response to stimulation with CD1b-expressing BMDC. This polarized cytokine production profile has also been observed for most of the group 1 CD1-restricted T-cell lines isolated from humans and hCD1Tg mice.3,14,17 However, HJ1 T cells are able to produce IL-4 in addition to IFN-γ and IL-17A (supplemental Figure 8) on stimulation with anti-CD3, indicating that these T cells have an intrinsic capability to produce cytokines associated with Th1, Th2 and even Th17 cells. This would suggest that CD1b-autoreactive T cells could play either a protective or pathogenic role in different disease settings, depending on the nature of the signals received during activation.
The basal autoreactivity of HJ1 T cells is significantly enhanced on stimulation with TLR agonist-treated or LM-infected DC. This enhanced HJ1 T-cell autoreactivity can be blocked by the addition of anti–IL-12p40, highlighting the role of proinflammatory cytokines in autoreactive CD1b-restricted T-cell activation. As DC express high levels of group 1 CD1 molecules, our findings suggest that CD1b-autoreactive HJ1 T cells may become fully activated during the early stages of bacterial infection and contribute to host defense against microbial pathogens. Consistent with this hypothesis, we found that the adoptive transfer of HJ1 T cells reduces bacterial burden in recipient mice infected with LM at day 3 after infection. Although the mechanism(s) by which HJ1 T cells contribute to this protective immunity remain to be defined, it is possible that HJ1 T cells could mediate anti-listerial effects through early production of IFN-γ to activate macrophages and DC and/or through production of IL-17A to recruit neutrophils. In addition, HJ1 T cells are cytolytic and may directly lyse LM-infected CD1b-expressing cells.
Besides autoreactive group 1 CD1-restricted T cells, several mycobacterial lipid-specific group 1 CD1-restricted T cells have been isolated from both humans and hCD1Tg mice.3,14 However, it is not clear whether these mycobacterial-specific T cells exhibit an activated phenotype before antigenic challenge. The dynamics of mycobacterial lipid-specific group 1 CD1-restricted T-cell responses have been characterized in hCD1Tg mice and, in contrast to iNKT-cell responses, display kinetics similar to that of conventional T cells.14 In addition, human mycobacterial-specific group 1 CD1-restricted T cells display a memory response, characteristic of adaptive immunity.45 Therefore, the group 1 CD1-restricted T-cell population appears to consist of both conventional-like T cells that participate in adaptive immune responses through recognition of specific microbial lipids and innate-like T cells that exhibit a pre-activated phenotype and are capable of self lipid-reactivity.
Much of our knowledge regarding CD1-mediated immunity has come from the study of CD1d and iNKT cells. While rodents lack group 1 CD1, other mammals possess various orthologs of CD1A, -B, or -C.3 The retention of large gene families encoding CD1 proteins in mammals suggests that each CD1 protein contributes a nonredundant function to immune responses. Our demonstration that CD1b-autoreactive HJ1 T cells contribute to protective immunity against LM infection provides the first direct evidence that autoreactive group 1 CD1-restricted T cells play a role in host defense against bacterial infection. The finding that the HJ1 T cells can be activated in vivo during bacterial infection raises the possibility that infection may trigger autoimmunity mediated by CD1b-autoreactive T cells. HJ1Tg/hCD1Tg mice reconstitute two key components of the human CD1b system in a mouse, which could be used to address the functional potential of CD1b-autoreactive T cells in various infection and autoimmune disease settings. This information would be helpful in the development of novel therapies or vaccines that target this unique T-cell population.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Stephen Balk for providing C1R and CD1b-expressing C1R transfectants; Dr Michael Brenner for purified anti-CD1b and anti-CD1c mAb; Dr Albert Bendelac for providing Vα14Tg mice; the National Institutes of Health tetramer core facility for CD1d tetramer and purified CD1b protein; the University of Chicago Transgenic Core Facility for microinjection of HJ1 TCR constructs; and Sharmila Shanmuganad for technical assistance.
This work is supported by National Institutes of Health grant R01 AI057460 to C.-R.W.
National Institutes of Health
Authorship
Contribution: S.L, H.-J.C., and K.F. performed the experiments; S.L. and C.-R.W. designed the experiments and analyzed the data; S.L., K.F., and C.-R.W. prepared the manuscript; and C.-R.W. supervised the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Chyung-Ru Wang, Department of Microbiology and Immunology, Northwestern University, 320 E Superior St, Searle 3-401, Chicago, IL 60611; e-mail: chyung-ru-wang@northwestern.edu.