Abstract
Mantle cell lymphoma (MCL) usually responds well to initial therapy but is prone to relapses with chemoresistant disease, indicating the need for novel therapeutic approaches. Inhibition of the p53 E3 ligase human homolog of the murine double minute protein-2 (HDM-2) with MI-63 has been validated as one such strategy in wild-type (wt) p53 models, and our genomic and proteomic analyses demonstrated that MI-63 suppressed the expression of the ribonucleotide reductase (RNR) subunit M2 (RRM2). This effect occurred in association with induction of p21 and cell-cycle arrest at G1/S and prompted us to examine combinations with the RNR inhibitor 2′,2′-difluoro-2′-deoxycytidine (gemcitabine). The regimen of MI-63–gemcitabine induced enhanced, synergistic antiproliferative, and proapoptotic effects in wtp53 MCL cell lines. Addition of exogenous dNTPs reversed this effect, whereas shRNA-mediated inhibition of RRM2 was sufficient to induce synergy with gemcitabine. Combination therapy of MCL murine xenografts with gemcitabine and MI-219, the in vivo analog of MI-63, resulted in enhanced antitumor activity. Finally, synergy was seen with MI-63–gemcitabine in primary patient samples that were found to express high levels of RRM2 compared with MCL cell lines. These findings provide a framework for translation of the rational combination of an HDM-2 and RNR inhibitor to the clinic for patients with relapsed wtp53 MCL.
Introduction
Mantle cell lymphoma (MCL) is a B-cell lymphoma that accounts for 6% to 8% of lymphoid malignancies and typically harbors the t(11;14) translocation, resulting in aberrant cyclin D1 expression and cell-cycle dysregulation.1 MCL patients present with a spectrum of disease types ranging from slow, indolent growing malignancies to more aggressive variants, such as the blastoid phenotype. It is characterized clinically by good initial responses to induction chemotherapy, but later it almost invariably relapses with a more aggressive course,1 making it an attractive model for novel drug development.2,3 One such novel agent is the proteasome inhibitor bortezomib that was shown to be active against MCL in a phase 1 trial4 and then approved for treatment of relapsed disease after multicenter studies fully demonstrated its activity.5-7 Other drugs that have shown promise against MCL include novel agents such as the mammalian target of rapamycin inhibitors temsirolimus8 and everolimus,9 the immunomodulatory drug lenalidomide,10,11 and more traditional cytotoxics such as (2′,2′-difluoro-2′-deoxycytidine (gemcitabine, dFdC).12,13
dFdC is an effective, broad-spectrum anticancer agent with activity in many malignancies, including as a single agent and in combination for non-Hodgkin lymphoma.14 It is transported into the cell mainly through the human equilibrative nucleoside transporter (hENT)-1,15 where it is metabolized by deoxycytidine kinase (dCK) into its 2 active forms, dFdC-diphosphate and dFdC-triphosphate (dFdCTP).16 The cytotoxic effects of dFdC are because of the ability of dFdC-diphosphate to inhibit the function of ribonucleotide reductase (RNR) and of dFdCTP to compete with dCTP for incorporation into DNA.17-19 dFdC-phosphate is identified by RNR as a substrate, metabolized within the active site, and then generates mutated products that inactivate the R1 subunit (RRM1) and induce the loss of the tyrosyl radical essential for action of the R2 subunit (RRM2). This produces a global decrease in the level of available cellular dNTPs for DNA replication. In addition, the dFdCTP moiety competes with cellular dCTP for insertion into DNA, after which DNA chain termination occurs and replication ceases.16 dFdC is highly effective in part because of this dual action, particularly because RNR overexpression is correlated with enhanced invasive potential, malignant transformation, and metastasis,20 and because overexpression by malignant cells conveys a selectivity that helps to reduce toxicity to normal cells.
Another attractive target for MCL therapy may be the p53 pathway, because DNA damage responses are altered in up to 75% of cases, in part through mutations of ataxia telangiectasia mutated21 or p53.21,22 Overexpression of the human homolog of the murine double minute protein-2 (HDM-2), which is seen in 22% or more of MCL cases, has also been associated with a more aggressive course and decreased survival.23-28 This may be due in part to its ability to decrease p53 levels through its activity as the major E3 ubiquitin ligase responsible for p53 ubiquitination before its proteasome-mediated degradation.29 Consistent with the possibility that nongenotoxic stabilization of p53 may be a valid approach to therapy of MCL, we and others have demonstrated previously the activity of HDM-2 inhibitors such as the Nutlins, and MI-63 and MI-219 against wild-type (wt) p53 MCL models both in vitro and in vivo.30-33 In this current work, we report the development of a mechanism-based combination regimen, which started with the observation that MI-63 decreased the expression levels of RRM2. By combining an HDM-2 inhibitor with dFdC, we were able to demonstrate that this regimen showed enhanced activity against MCL cell lines and xenografts compared with either agent alone. This approach may prove fruitful for the treatment of patients with relapsed, wtp53 MCL, and is ready for translation to the clinic.
Methods
Reagents
The HDM-2 inhibitors MI-63 and MI-219 were provided by Ascenta Therapeutics. Nutlin-3, dFdC for in vitro work, doxorubicin, and deoxyribonucleotides (dNTPs) were purchased from Sigma-Aldrich, whereas dFdC for in vivo work was from the M. D. Anderson Cancer Center Clinical Pharmacy.
Cell culture and patient samples
JVM-2, Granta-519, REC-1, and JeKo-1 MCL cell lines were purchased from the German Collection of Microorganisms and Cell Cultures and validated through The M. D. Anderson Cancer Center Characterized Cell Line Core Facility. Cells were grown in RPMI 1640 (Invitrogen) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich). Primary patient samples were obtained from the M. D. Anderson Department of Lymphoma and Myeloma Satellite Lymphoma Tissue Bank. Informed consent was obtained from each patient in compliance with the Declaration of Helsinki according to an M. D. Anderson Cancer Center Institutional Review Board-approved protocol, and samples were purified as described previously.30
Semiquantitative and real-time PCR
Total RNA was isolated using an RNeasy Plus kit (QIAGEN), and cDNA was synthesized using SuperScript II (Invitrogen). PCR was performed for 30 cycles annealing at 54.4°C for RRM2 (GenBank accession NM_001165931) using sense primer 5′-GAAGGCAGAGGCTTCCTTTT-3′ and antisense primer 5′-AGAAACAGCGGGCTTCTGTA-3′ and for the p53-inducible R2 subunit (R2p53, or p53-inducible RNR subunit M2B [RRM2B]; GenBank accession NM_015713) using sense primer 5′-GGGGATTCTGTGGTCAGATG-3′ and antisense primer 5′-GGCCAGCTTTTTCCAATCTT-3′. Primers and conditions for β2-microglobulin (β2M), which was used as a control, were as described previously.34 Real-time PCR for RRM1, RRM2, RRM2B, dCK, and hENT-1 was performed on a StepOne PCR analyzer (Applied Biosystems) using inventoried real-time TaqMan-FAM and GAPDH-VIC probes. Relative transcript expression (RQ) was determined using JVM-2 cells as a calibrator using the ΔΔCT method according to the manufacturer's instructions (Applied Biosystems).
p53 sequencing
Genomic DNA was extracted from MCL patient samples, and all exons, introns, and regulatory regions of p53 were sequenced by the M. D. Anderson DNA Analysis Core Facility. Exons were amplified using custom PCR primers, and Sanger sequencing was performed on a 3730xl DNA analyzer using BigDye Terminator v3 chemistry (Applied Biosystems). Mutations analysis was performed using SeqScape Version 2.5 software (Applied Biosystems).
Gene expression profiling
RNA was extracted from MI-63– or vehicle-treated cells using TRIzol (Invitrogen) in triplicate, and RNA was submitted to Genome Explorations for gene expression profiling analysis using the Affymetrix Human Genome U133 Plus 2.0 array platform.
Immunoblotting
Protein expression was measured by immunoblot analysis performed as described previously.34 Antibodies to RRM1, RRM2, RRM2B, and p53 were purchased from Santa Cruz Biotechnology, the anti-poly(ADP-ribose) polymerase (PARP) antibody was from Cell Signaling Technology, anti-p21 and anti–HDM-2 antibodies were purchased from EMD4Biosciences, and the anti–β-actin antibody was from Sigma-Aldrich.
Cell-cycle and cell-death analysis
Cells were treated with drug for 24 hours and then fixed in 70% ethanol and stained with propidium iodide (Sigma-Aldrich). Cell-cycle data were analyzed on a CANTO II flow cytometer (BD Biosciences) using FlowJo Version 7.6.1 (TreeStar). Cell death also was measured using annexin-V Pacific Blue and TO-PRO-3 (Invitrogen) on a CANTO II flow cytometer using FlowJo Version 7.6.1.
Cell-proliferation assay
The WST-1 reagent (Roche Diagnostics) was used to determine the effects of chemotherapy agents on cell proliferation and to calculate their relative IC50 values, as published previously.35
Lentiviral shRNA knockdown of RRM2
Lentiviral shRNA particles (Santa Cruz Biotechnology) containing either a scrambled sequence shRNA or shRNAs targeting RRM2 were transduced overnight at a multiplicity of infection of 2 into REC-1 MCL cells. These cells were then drug selected with 2 μg/mL puromycin (Sigma-Aldrich) to generate stable cell lines.
Drug synergy assays
To evaluate for the presence of synergistic interactions, the methods of Chou and Talalay were used as described previously.36,37 In brief, MI-63 or dFdC was added to cells, and the IC50 value of each drug individually was determined using the WST-1 assay. A range of serial dilutions was made across the IC50 dose range, with the IC50 set as 1×, and dilutions were made relative to this value. The agents were then added simultaneously to the cells or in a sequence-specific order for 3 days, and WST-1 assays were performed. Data were then analyzed using CalcuSyn Version 2 software (Biosoft), and combination indices (CIs) were calculated.
In vivo xenograft model
Experiments were performed in accordance with procedures and protocols approved by the M. D. Anderson Cancer Center Animal Care and Use Committee. Six- to 8-week-old male CB-17 severe combined immunodeficiency mice (Harlan) were inoculated in the right flank subcutaneously with 5 × 106 Granta-519 cells. Tumor burden was monitored by measuring the length and width of visible masses using calipers 3 times per week, and tumor volumes were calculated using the equation 0.4 × L × W (L indicates length; W, width).2 When tumor burdens reached ∼100 mm3 in volume, mice were randomized into 4 groups of 5 mice each to intraperitoneal injections of vehicle (10% polyethylene glycol, 3% Cremophor EL [Sigma-Aldrich], and 87% phosphate-buffered saline), MI-219 (100 mg/kg), dFdC (60 mg/kg), or both agents simultaneously at 50% of the single-agent dose (50 mg/kg MI-219; 30 mg/kg dFdC). Mice were killed by CO2 asphyxiation when tumor size reached > 15 mm in any 1 direction according to institutional guidelines.
Statistical analyses
Data were subjected to statistical analyses by calculation of the SEM. The significance of drug–effect relationships was further determined using 1-tailed unpaired t tests using Excel 2007 software (Microsoft). For the in vivo studies, an analysis of cooperative effects of MI-219 and dFdC on tumor growth data were performed using a Bayesian bootstrapping approach. We calculated Pr(min(μM,μG) < μC data), the posterior probability that the minimum of the 2 (posterior) means of the response, as evidenced by either final tumor volume, or average tumor growth rate, for MI-219 alone, μM, or dFdC alone, μG, was less than the (posterior) mean response for the combination, μC. This probability calculates the likelihood that average response in the combination arm will be less than the minimum of the 2 single agent arms. Cooperative effects are shown if this posterior probability is large. The statistical software R Version 2.120.0 (www.r-project.org) was used for all the analyses using 10 000 bootstrap samples.
Results
MI-63 induces down-regulation of RRM2 in MCL cells
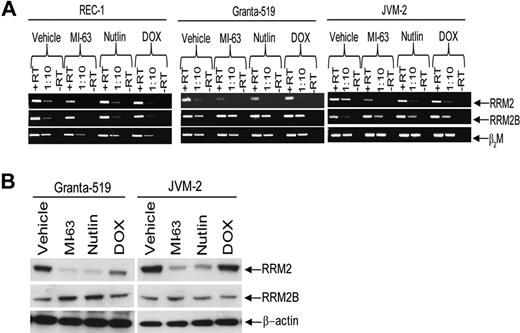
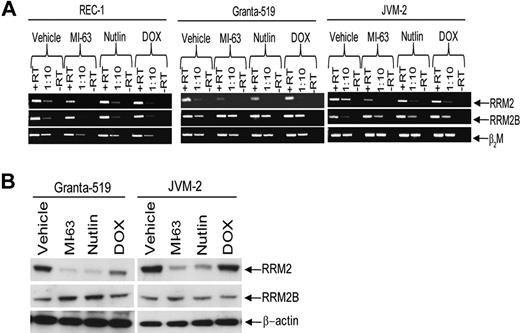
We demonstrated previously that inhibition of HDM-2 by MI-63 was an effective strategy against MCL,30 and sought to further explore the consequences of HDM-2 inhibition at the genomic level. Gene expression profiling of wtp53 REC-1 cells exposed to MI-63 for 24 hours revealed enhanced expression of known p53 target genes (Table 1), such as p21 and Sestrin-1. In addition, strong up-regulation of the apoptotic mediators tumor necrosis factor receptor superfamily, member 6 (Fas), and Bcl-2–associated X protein (Bax) was seen, consistent with stabilization of p53 by MI-63. This coincided with down-regulation of key genes involved in cell-cycle progression and cell proliferation, such as E2F transcription factor 8 (E2F8) and thymidine kinase. Of particular interest was the suppression of RRM2, which was reduced by more than 3-fold based on 2 different probes relative to the vehicle control. To further confirm this decrease, we measured RRM2 transcript levels by RT-PCR in Granta-519 and JVM-2, 2 other wtp53 MCL cell lines. Compared with β2M as a control, exposure of REC-1, Granta-519, or JVM-2 cells to either MI-63 or the cis-imidazoline HDM-2 inhibitor Nutlin resulted in decreased RRM2 transcript levels in all cell lines (Figure 1A). Conversely, and as expected, HDM-2 inhibition increased transcript levels for RRM2B, which supplies excess dNTPs to cells undergoing genotoxic stress.38 Suppression of transcription of RRM2 also resulted in a profound decrease in RRM2 expression at the protein level in Granta-519 and JVM-2 cells (Figure 1B) compared with β-actin as a control, and compared with RRM2B, which increased modestly after exposure to MI-63.
HDM-2 inhibitors decrease RRM2 polypeptide expression. (A) REC-1, Granta-519, and JVM-2 wtp53 MCL cell lines were treated for 24 hours with either vehicle, 5μM MI-63, 5μM Nutlin, or 0.5μM doxorubicin (DOX) as a positive control for 24 hours. Qualitative PCR was performed to detect RRM2 and RRM2B mRNA levels, as well as β2M as a loading control, and transcripts were visualized by native gel electrophoresis. Lanes marked +RT received 1 μL of cDNA stock solution, whereas those labeled with 1:10 received 1 μL of 1:10 dilution of +RT. Representative images are shown in both panels of 1 of 3 independent experiments. (B) Protein levels of RRM2 and RRM2B, as well as β-actin as a loading control, were determined in lysates from MCL cells treated as described in panel A.
HDM-2 inhibitors decrease RRM2 polypeptide expression. (A) REC-1, Granta-519, and JVM-2 wtp53 MCL cell lines were treated for 24 hours with either vehicle, 5μM MI-63, 5μM Nutlin, or 0.5μM doxorubicin (DOX) as a positive control for 24 hours. Qualitative PCR was performed to detect RRM2 and RRM2B mRNA levels, as well as β2M as a loading control, and transcripts were visualized by native gel electrophoresis. Lanes marked +RT received 1 μL of cDNA stock solution, whereas those labeled with 1:10 received 1 μL of 1:10 dilution of +RT. Representative images are shown in both panels of 1 of 3 independent experiments. (B) Protein levels of RRM2 and RRM2B, as well as β-actin as a loading control, were determined in lysates from MCL cells treated as described in panel A.
dFdC is synergistic with MI-63
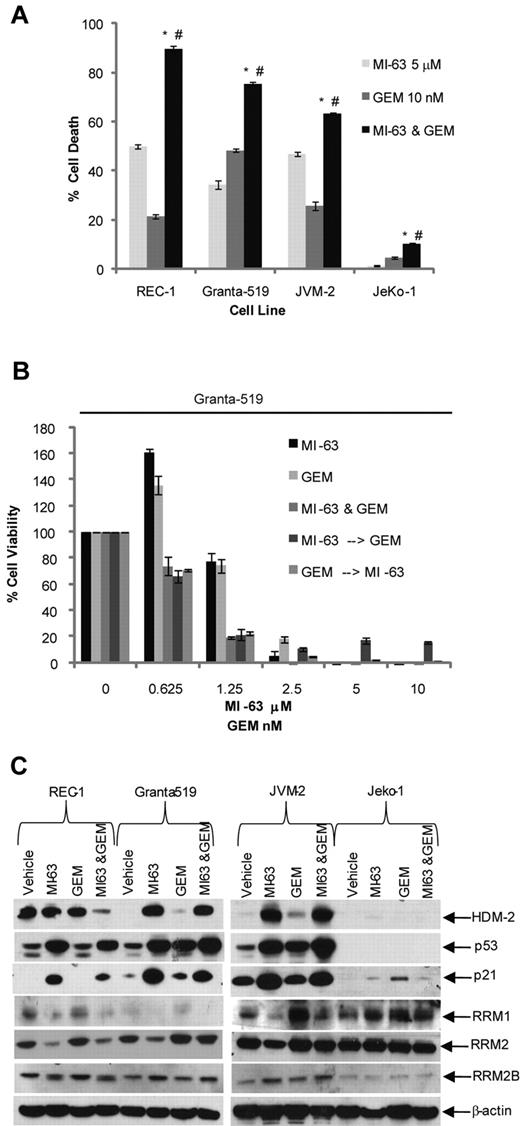
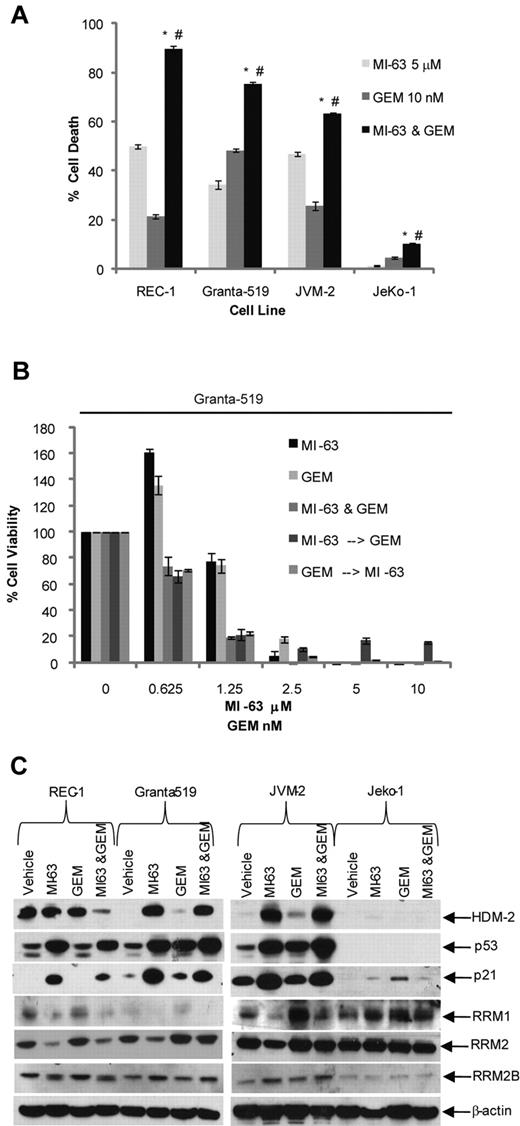
We next considered the possibility that MI-63–mediated suppression of RRM2 could enhance sensitivity to dFdC, because RRM2 overexpression correlates with enhanced clinical dFdC resistance.13 Exposure of MCL cell lines to MI-63 alone at 5μM for 24 hours resulted in the induction of cell death in 35% to 50% of wtp53 cells (Figure 2A), whereas dFdC at 10nM produced apoptosis in 20% to 50% of cells. When both agents were added simultaneously for 24 hours, this increased the proportion of cells undergoing cell death to 90% in REC-1, 80% in Granta-519, and 60% in JVM-2 cells. Statistical analysis using an unpaired t test indicated that the combination of MI-63 and dFdC induced a significantly greater reduction in viability (P < .05) compared with either MI-63 or dFdC alone. The sole exception to this pattern were mutant (mut) p53 JeKo-1 cells, in which small amounts of cell death were seen because of their relative resistance to low doses of dFdC and complete lack of sensitivity to MI-63.
dFdC and MI-63 enhance cell death. (A) REC-1, Granta-519, and JVM-2, and the mutp53 MCL cell line JeKo-1, were treated for 24 hours with vehicle, 5μM MI-63, 10nM dFdC (GEM), or both. Flow cytometric analysis was then performed after staining with annexin-V and TO-PRO-3, from which the proportion of cells undergoing apoptosis was calculated and normalized to the vehicle control group. Values represent the mean ± SE from 3 independent experiments. An unpaired t test was performed to evaluate for significance; *P < .01 relative to MI-63 alone; #P < .01 relative to dFdC alone. (B) Granta-519 cells were incubated simultaneously with single agent MI-63 or dFdC for 72 hours. In parallel, cells were exposed either first to MI-63 for 24 hours followed by dFdC and MI-63 for 48 hours or to dFdC first for 24 hours followed later by MI-63 and dFdC for 48 hours. Cell viability was determined using the WST-1 reagent, and results are expressed as the percentage viability relative to the vehicle control, which was arbitrarily set at 100%. The presence of synergistic interactions was determined by calculation of the CI from the cell viabilities calculated across a serial dilution range of MI-63 or dFdC (Table 2). Each panel provides representative data from 1 of 3 independent experiments. (C) Protein levels of HDM-2, p53, RRM1, RRM2, RRM2B, and p21, and β-actin as a loading control, were determined by Western blotting of cellular lysates.
dFdC and MI-63 enhance cell death. (A) REC-1, Granta-519, and JVM-2, and the mutp53 MCL cell line JeKo-1, were treated for 24 hours with vehicle, 5μM MI-63, 10nM dFdC (GEM), or both. Flow cytometric analysis was then performed after staining with annexin-V and TO-PRO-3, from which the proportion of cells undergoing apoptosis was calculated and normalized to the vehicle control group. Values represent the mean ± SE from 3 independent experiments. An unpaired t test was performed to evaluate for significance; *P < .01 relative to MI-63 alone; #P < .01 relative to dFdC alone. (B) Granta-519 cells were incubated simultaneously with single agent MI-63 or dFdC for 72 hours. In parallel, cells were exposed either first to MI-63 for 24 hours followed by dFdC and MI-63 for 48 hours or to dFdC first for 24 hours followed later by MI-63 and dFdC for 48 hours. Cell viability was determined using the WST-1 reagent, and results are expressed as the percentage viability relative to the vehicle control, which was arbitrarily set at 100%. The presence of synergistic interactions was determined by calculation of the CI from the cell viabilities calculated across a serial dilution range of MI-63 or dFdC (Table 2). Each panel provides representative data from 1 of 3 independent experiments. (C) Protein levels of HDM-2, p53, RRM1, RRM2, RRM2B, and p21, and β-actin as a loading control, were determined by Western blotting of cellular lysates.
Combination chemotherapy regimens can have different efficacies depending on the sequence of addition of the drugs of interest. We therefore titrated MI-63 and dFdC against a panel of MCL cell lines and sought to determine what sequence of administration could be superior. We first determined the IC50 value of each cell line with both MI-63 and dFdC at 3 days (supplementa1 Figure 1A and B, respectively, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These calculated IC50 values allowed us to dose the agents in a 1:1 ratio based on the fold IC50 (0.25, 0.5, 1, 2, and 4×), as suggested for synergy calculations as set out in the methods of Chou and Talay.36,37 Simultaneous administration of the 2 agents was compared with a schedule that allowed for pretreatment with 1 agent for 24 hours, followed by 48 hours with the second agent, for a total time of 72 hours. In Granta-519 cells, all 3 of these approaches resulted in enhanced cell killing compared with either agent alone at the lower drug concentrations (Figure 3B). CI analysis yielded CI values of 0.32 to 0.970, consistent with the presence of strong synergy for all 3 sequences (Table 2). Simultaneous addition of MI-63 and dFdC also showed synergy in REC-1 (CI, 0.33-0.93) and JVM-2 cells (0.71-0.81; Table 2). Pretreatment with MI-63 was synergistic in all 3 cell lines, particularly at low concentrations of 0.25× up to 1× of the IC50, with CI values ranging as low as 0.28 in REC-1. However, synergy was lost at the higher concentrations tested of 2 and 4× the IC50. In addition, pretreatment with dFdC demonstrated synergy in REC-1 cells (CI, 0.32-0.74), although this was lost at higher concentrations. In contrast, JVM-2 and Granta-519 cells showed synergy across the concentration range, with a CI range of 0.67 to 0.91 and 0.41 to 0.97, respectively.
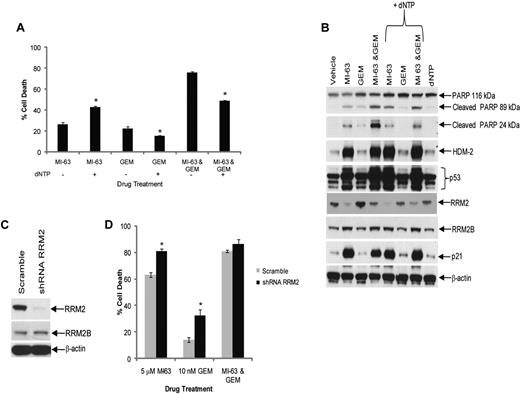
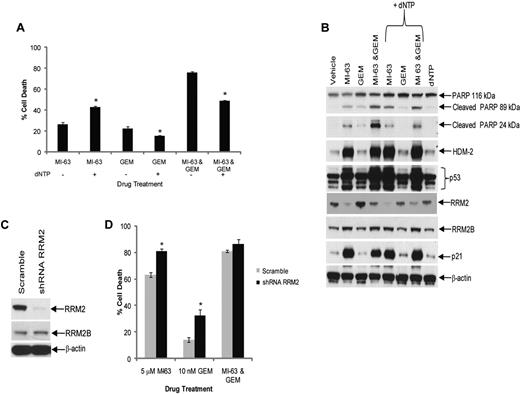
Excess dNTP reverses synergy between MI-63 and dFdC. (A) Granta-519 cells were incubated with vehicle, 5μM MI-63, 10nM dFdC (GEM), or both agents simultaneously for 24 hours, either without or with exogenous dNTPs at 50μM. Cell death was then determined by flow cytometry using annexin-V and TO-PRO-3 staining relative to the vehicle control. Each panel provides representative data from 1 of 3 independent experiments. An unpaired t test was performed comparing cells to which dNTPs had been added to those exposed to drug alone; *P < .05. (B) Cellular lysates were probed for PARP, HDM-2, p53, RRM2, RRM2B, and p21, as well as β-actin as a loading control. (C) REC-1 cells were infected with Lentiviral particles carrying a scrambled sequence shRNA or an shRNA targeting RRM2, and stable cell lines were generated by drug selection. Cellular lysates were then probed for their content of RRM2, RRM2B, and β-actin as a loading control. (D) REC-1 shRNA cells were incubated with vehicle, 5μM MI-63, 10nM dFdC, or both agents simultaneously for 24 hours, and the proportion of cells undergoing apoptosis was determined by flow cytometry using annexin-V and TO-PRO-3. Statistically significant differences are defined as *P < .05.
Excess dNTP reverses synergy between MI-63 and dFdC. (A) Granta-519 cells were incubated with vehicle, 5μM MI-63, 10nM dFdC (GEM), or both agents simultaneously for 24 hours, either without or with exogenous dNTPs at 50μM. Cell death was then determined by flow cytometry using annexin-V and TO-PRO-3 staining relative to the vehicle control. Each panel provides representative data from 1 of 3 independent experiments. An unpaired t test was performed comparing cells to which dNTPs had been added to those exposed to drug alone; *P < .05. (B) Cellular lysates were probed for PARP, HDM-2, p53, RRM2, RRM2B, and p21, as well as β-actin as a loading control. (C) REC-1 cells were infected with Lentiviral particles carrying a scrambled sequence shRNA or an shRNA targeting RRM2, and stable cell lines were generated by drug selection. Cellular lysates were then probed for their content of RRM2, RRM2B, and β-actin as a loading control. (D) REC-1 shRNA cells were incubated with vehicle, 5μM MI-63, 10nM dFdC, or both agents simultaneously for 24 hours, and the proportion of cells undergoing apoptosis was determined by flow cytometry using annexin-V and TO-PRO-3. Statistically significant differences are defined as *P < .05.
Expression of RNR is regulated in a cell cycle–dependent manner,39,40 and it was therefore of interest to analyze the impact of simultaneous addition of MI-63 and dFdC on wtp53 MCL cells. Cell-cycle analysis indicated that MI-63 as a single agent induced a G1 cell-cycle arrest (supplemental Figure 2), whereas dFdC induced a predominantly S-phase arrest. When both agents were combined, they generated a G1 arrest in REC-1 and JVM-2 cells and a G1/S arrest in Granta-519 cells. These findings suggest that MI-63 was able to override the S-phase arrest induced by dFdC and that the loss of RRM2 expression was because of a G1 cell-cycle arrest, during which this subunit is known to be depleted due in part to its short half-life.39 At the protein level, MI-63 as a single agent induced an accumulation of p53, p21, and HDM-2 in all 3 wtp53 MCL cell lines compared with the vehicle control (Figure 2C). The same was true for the MI-63–dFdC combination, although the levels of these targets typically did not quite reach those seen with MI-63 alone. Enhanced levels of p21 would be expected to repress cyclin-dependent kinase 2, which would cause hypophosphorylation of Retinoblastoma protein and E2F, the latter of which is necessary for RRM2 transcription,39,40 providing another mechanism for reduced RRM2 expression. Consistent with this mechanism, MI-63 alone decreased RRM2 levels in all 3 wtp53 MCL cell lines (Figure 2C), whereas dFdC seemed to mildly induce RRM2, suggesting the involvement of an inducible chemoresistance pathway. When the 2 were combined, cells exposed to the MI-63–dFdC regimen showed reduced levels of RRM2 compared with dFdC alone, possibly accounting for the enhanced proapoptotic activity.
Synergy between MI-63 and dFdC is related to RRM2
HDM-2 inhibition triggers a p53-dependent death program, and because dFdC also activates apoptotic signaling in part through p53,41 it was possible that the MI-63–dFdC combination was working through enhanced p53 induction. Western blotting of MCL cell lines treated with the combination did not reveal increased levels of p53, p21, or HDM-2 compared with either MI-63 or dFdC alone (Figure 2C), suggesting this was not the case. However, to test this more directly, we added an excess of dNTPs to the culture media of cells exposed to either agent or the combination, and then we evaluated the effects on cell death and signaling. Addition of dNTPs to MI-63–treated cells did not reduce cell death (Figure 3A), which was actually increased (P = .003), whereas dNTPs slightly attenuated the degree of cell death induced by dFdC alone (P = .03). When dNTPs were added to cells exposed to the combination, the synergistic effect was substantially blunted, with a reduction in cell death from 75% to 50% (P < .01), which was comparable with that induced by MI-63 alone (Figure 3A). This was associated with a decreased level of cleavage of PARP in the presence of dNTPs compared with that without supplemental nucleotides (Figure 3B). Notably, the levels of p53, p21, and HDM-2 that accumulated after exposure to the MI-63–dFdC combination were not dramatically changed by the presence of dNTPs, suggesting that p53 signaling was not affected. To further evaluate the role of suppression of RRM2 in the synergy of this combination, we generated REC-1 cells in which expression of this polypeptide was reduced by > 90% using a targeted shRNA (Figure 3C). Knockdown of RRM2 enhanced cell death to some extent because of both MI-63 and dFdC (Figure 3D), but the combination regimen was no longer able to show synergistic induction of apoptosis. These findings support the possibility that the MI-63–dFdC regimen is active through the ability of the HDM-2 inhibitor to reduce RRM2, thereby sensitizing to the ribonucleotide reductase inhibitor.
Activity of MI-63–dFdC against an MCL xenograft model
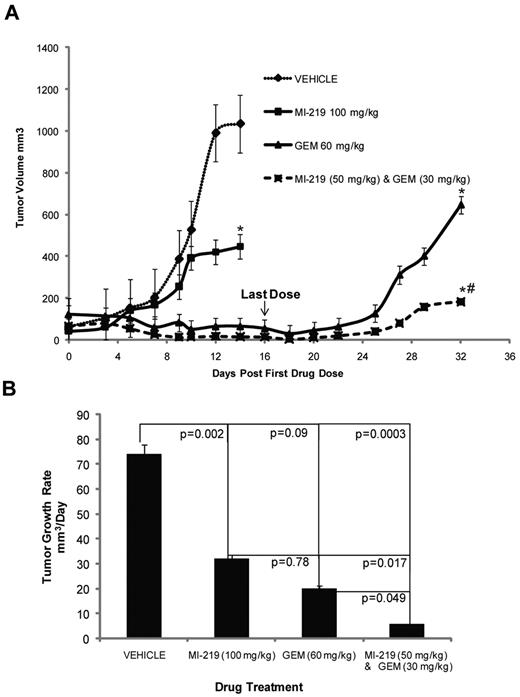
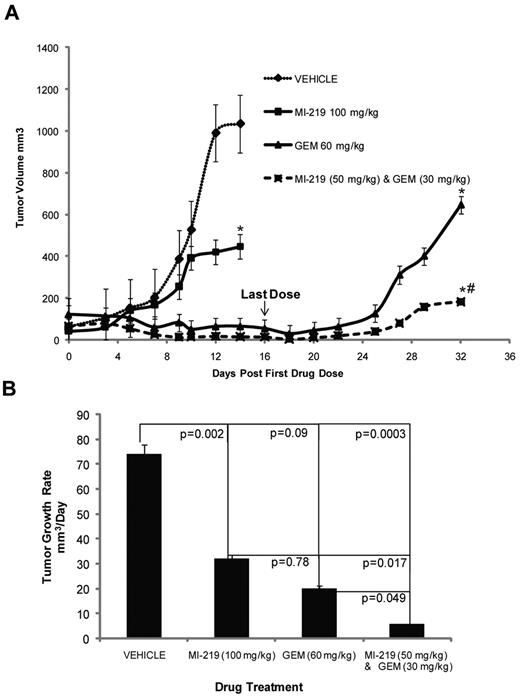
Because the combination of MI-63 and dFdC showed promising efficacy in vitro, we evaluated its activity against established MCL xenografts in an immunodeficient murine model using MI-219, the in vivo formulation of MI-63.42 The latter agent alone, when given daily for 2 weeks at a dose of 100 mg/kg, slowed tumor growth (P < .05; Figure 4 top panel) to an average of 30 mm3/day (Figure 4 bottom panel), compared with 74 mm3/day for the vehicle control (P = .002). dFdC as a single agent, given at 60 mg/kg every 3 days for a total of only 2 weeks, also delayed tumor growth (P < .05; Figure 4 top panel), slowing it to 20 mm3/day (P = .09; Figure 4 bottom panel). For the combination treatment, each agent was reduced in dose by 50%, and MI-219 at 50 mg/kg with dFdC at 30 mg/kg for 2 weeks slowed tumor growth to 5 mm3/day (P = .049). Analysis of the cooperative effects between MI-219 and dFdC in terms of the final tumor volume indicated that the posterior probability of cooperative effect was found to be 0.9951, indicating that there was less than a 50 in 10 000 chance that the combination did not have a cooperative effect. For the average tumor growth rate, the cooperative effect was > 0.9977, demonstrating that there was less than a 25 in 10 000 chance that the combination did not have a cooperative effect, and a > 9975 in 10 000 chance that such an effect did exist. These analyses indicated a high level of evidence for the presence of cooperative effects of the combination treatment compared with the single agents. No toxicities were observed with either the single agents or the combination at the doses used in these cohorts.
MI-63 and dFdC inhibit tumor growth in vivo. (A) Severe combined immunodeficiency mice were inoculated with Granta-519 cells subcutaneously and monitored until tumors were established. Five mice per group were then injected intraperitoneally with vehicle, MI-219 daily for 2 weeks, dFdC (GEM) every third day for 2 weeks, or both agents using the same schedules but at a 50% dose reduction. Tumor volumes were measured 3 times per week and are plotted as a function of time in the top panel. Statistically significant differences are defined as *P < .05 relative to the vehicle control and as #P < .05 relative to MI-219 alone. In the bottom panel, the average tumor growth rate per day was calculated, and the P values of each group are shown relative to the vehicle group, as well as to MI-219 alone, or dFdC alone.
MI-63 and dFdC inhibit tumor growth in vivo. (A) Severe combined immunodeficiency mice were inoculated with Granta-519 cells subcutaneously and monitored until tumors were established. Five mice per group were then injected intraperitoneally with vehicle, MI-219 daily for 2 weeks, dFdC (GEM) every third day for 2 weeks, or both agents using the same schedules but at a 50% dose reduction. Tumor volumes were measured 3 times per week and are plotted as a function of time in the top panel. Statistically significant differences are defined as *P < .05 relative to the vehicle control and as #P < .05 relative to MI-219 alone. In the bottom panel, the average tumor growth rate per day was calculated, and the P values of each group are shown relative to the vehicle group, as well as to MI-219 alone, or dFdC alone.
MI-63 and dFdC are active against primary MCL cells
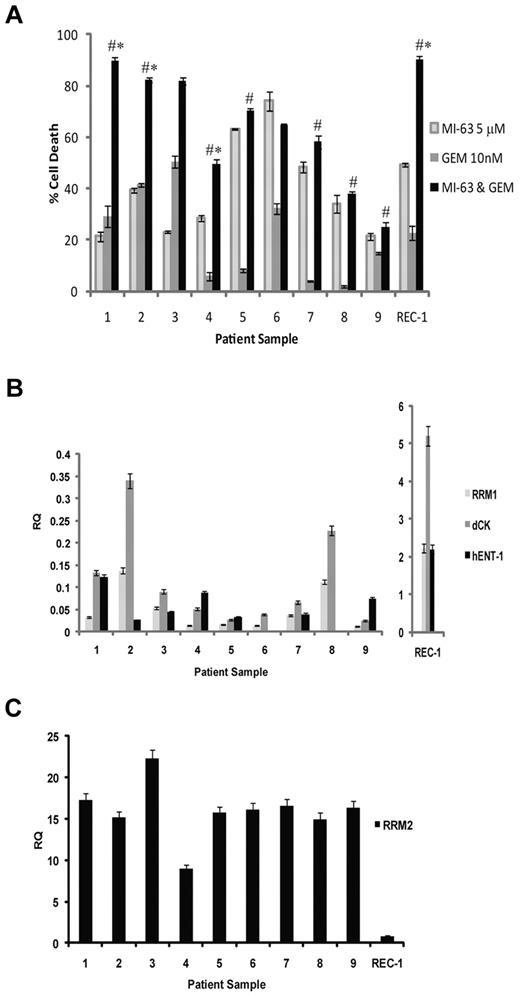
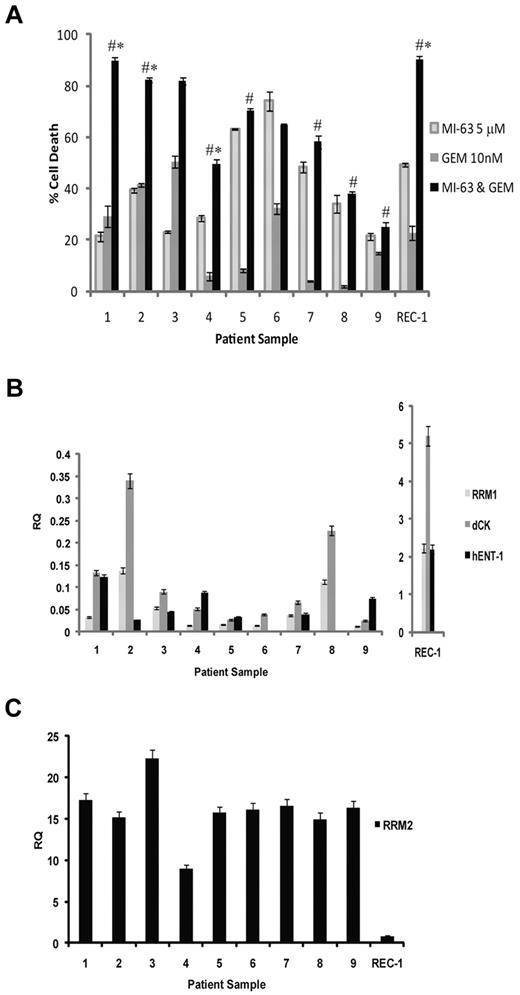
Regimens such as MI-63 and dFdC that show synergy preclinically may be attractive candidates for translation to the clinic; therefore, we evaluated the activity of this mechanism-based combination against patient-derived MCL cells. Nine primary samples were obtained, 6 of which harbored 2 wtp53 alleles based on genomic DNA sequencing, whereas 2 were heterozygous, with 1 wtp53 and 1 mutant p53 allele (supplemental Table 1). The majority of these samples were from patients with relapsed disease that had been previously treated with up to 4 prior lines of therapy (supplemental Table 1), whereas 2 were from patients with newly diagnosed disease. Exposure of these samples to MI-63 for 24 hours induced cell death in 20% to 80% of cells (Figure 5A). dFdC alone for 24 hours showed greater variability, in that although some samples were quite responsive, with cell death seen in up to 53%, such as the cells from patient 3, others were relatively resistant, with values as low as 3%, such as in patient 8. When the combination regimen was used for 24 hours, substantial enhanced effects consistent with synergy were seen in the samples from patients 1 to 4 (P < .05 relative to MI-63 alone or dFdC alone). Weakly additive effects were seen in patients 5, 7, and 9 (P < .05 relative to dFdC alone), whereas patients 6 and 8 showed no enhancement over what had been seen with MI-63 alone (Figure 5A).
Synergistic activity of MI-63 and dFdC in MCL patient samples. (A) MCL cells were purified from the peripheral blood of patients with circulating neoplastic cells using magnetic-activated cell sorting and CD19 microbeads. These cells were then either exposed to 5μM MI-63, 10nM dFdC, or both agents simultaneously for 24 hours. Cell death was determined by flow cytometry using annexin-V and TO-PRO-3 staining relative to the vehicle-treated control, and REC-1 cells were included as an additional control. Each panel provides representative data from 1 of 3 independent experiments, and *P < .05 denote significance relative to MI-63 alone, and #P < .05 denote significance relative to dFdC alone. (B) Aliquots of each of the primary samples analyzed in panel A also were subjected to RNA extraction, cDNA was synthesized, and the levels of RRM1, dCk, hENT-1, and RRM2 were measured by quantitative real-time PCR using the ΔΔCT method with the JVM-2 cell line used as a relative calibrator. The transcript level in REC-1 cells also was measured as a control and is plotted on a separate scale because of their high expression of RRM1, dCK, and hENT-1. (C) Real-time PCR analysis of RRM2 transcript levels in MCL patient samples is shown, along with REC-1 as a cell line control.
Synergistic activity of MI-63 and dFdC in MCL patient samples. (A) MCL cells were purified from the peripheral blood of patients with circulating neoplastic cells using magnetic-activated cell sorting and CD19 microbeads. These cells were then either exposed to 5μM MI-63, 10nM dFdC, or both agents simultaneously for 24 hours. Cell death was determined by flow cytometry using annexin-V and TO-PRO-3 staining relative to the vehicle-treated control, and REC-1 cells were included as an additional control. Each panel provides representative data from 1 of 3 independent experiments, and *P < .05 denote significance relative to MI-63 alone, and #P < .05 denote significance relative to dFdC alone. (B) Aliquots of each of the primary samples analyzed in panel A also were subjected to RNA extraction, cDNA was synthesized, and the levels of RRM1, dCk, hENT-1, and RRM2 were measured by quantitative real-time PCR using the ΔΔCT method with the JVM-2 cell line used as a relative calibrator. The transcript level in REC-1 cells also was measured as a control and is plotted on a separate scale because of their high expression of RRM1, dCK, and hENT-1. (C) Real-time PCR analysis of RRM2 transcript levels in MCL patient samples is shown, along with REC-1 as a cell line control.
To gain further insight into the sensitivity of these primary samples, we evaluated the expression levels of hENT-1; the dFdC transporter dCK, which activates and metabolizes dFdC; and RRM1 and RRM2 by quantitative real-time PCR. Primary samples 1 to 4 expressed both hENT-1 and dCK (Figure 5B), suggesting they could readily transport and metabolize dFdC and therefore respond well to the single-agent and combination therapy. Samples 5, 7, and 9, in contrast, had variable but generally lower expression levels of these genes (Figure 5B), possibly accounting for the lower level of enhanced cell death with the MI-63–dFdC regimen. Finally, samples 6 and 8, which showed no additive effects, also both expressed no hENT-1 (Figure 5B), although the former did show some sensitivity to single-agent dFdC (Figure 5A). All of the samples showed at least some expression of RRM1, but this expression was lower than that seen in some of the MCL cell lines (Figure 5B), and did not seem to correlate with sensitivity to dFdC. Similarly, RRM2 expression levels, which were more consistent between samples (Figure 5C), did not predict for sensitivity to either dFdC or MI-63–dFdC. However, in comparison with dCK, RRM1, and hENT-1 in the MCL cell lines, primary samples did express a relative abundance of RRM2 (Figure 5C). These findings support the translation of strategies to the clinic that suppress RRM2 levels in combination with dFdC, such as HDM-2 inhibition, for patients with relapsed mantle cell lymphoma that harbor wtp53 and express the human equilibrative nucleoside transporter and deoxycytidine kinase.
Discussion
MCL remains a challenging entity for the clinician, because there is as yet no widely accepted standard of care.1-3 Immunochemotherapy strategies incorporating rituximab are commonly used in the front-line settings.43,44 Younger patients may receive standard dose cyclophosphamide, doxorubicin, vincristine, and prednisone, followed by autologous stem cell transplantation, or more aggressive approaches with hyperfractionated cyclophosphamide regimens without transplantation.43,44 These more intense approaches seem to increase complete response rates and overall survivals, but they cannot be applied in all cases given that the median age at the time of diagnosis is typically in the mid-1960s. In the latter patients, and even in a substantial proportion of those who can receive aggressive therapy, the disease is still not cured, and at the time of relapse is more aggressive, less chemosensitive, and has a poor postrelapse survival.43,44 As a result, there is a strong imperative to develop new therapeutic agents and combinations and to also use them in a molecularly adapted manner, so that only those who would be most likely to benefit are exposed to the accompanying risks.
Our earlier preclinical studies had supported the possibility that HDM-2 inhibition was a rational strategy for therapy of MCL, and we therefore sought to develop combination regimens that could enhance this activity further. We first demonstrated that treatment of wtp53 MCL models with MI-63 resulted in a profound decrease in expression of RRM2 (Table 1; Figure 1). This was associated with an arrest at G1/S, and accumulation of p53 and its downstream target p21. RRM1, which is transcribed in S phase, has a long half-life of 20 hours, and remains at a constant level throughout the cell cycle.45 In contrast, whereas RRM2 is also regulated in a cell cycle–dependent manner, and transcribed during S phase, it has a much shorter half-life of 3 hours,46 and expression levels are therefore much more sensitive to interruption of its transcription and translation. The decrease in RRM2 with MI-63 indicated that the combination of MI-63 and dFdC could be synergistic, particularly because dFDCP is known to inhibit RRM2,12 and in lung cancer patients decreased levels of RRM2 conveyed sensitivity to dFdC.47 Consistent with this possibility, the combination of MI-63 and dFdC was effective against MCL, and reached the statistical criteria for synergistic interactions (Table 2). Furthermore, studies into the influence of the sequence dependence of dFdC and MI-63 demonstrated that simultaneous use of the 2 agents, as well as pretreatment with either one or the other, was effective (Figure 2). In addition, MI-63 seemed to overcome the S-phase arrest effect of dFdC, and the expected subsequent enhanced transcription of RRM2. A synergistic interaction was further supported by the MCL xenograft model, where a combination of MI-63 and dFdC showed enhanced tumor growth delay, even at a 50% dose reduction compared with either of the single agents (Figure 4).
Treatment of primary samples ex vivo also yielded promising results despite that 6 of the 9 patients had received prior dose-intensive chemotherapy in the form of either stem cell transplantation or hyperfractionated cyclophosphamide (supplemental Table 1). Notably, the efficacy against patient samples also showed some dependency on the expression of the nucleoside transporter hENT-1 and on dCK (Figure 5). Of particular interest was that the levels of hENT-1, dCK, and RRM1 were substantially higher in the MCL cell line REC-1 and lower in the MCL patient samples. Conversely, RRM2 levels were substantially higher in the primary lymphoma cells than in the REC-1 model, and both of these would be expected to negatively impact on the sensitivity of MCL in vivo to dFdC. Indeed, the expression of hENT-1 has been shown to be a mediator of sensitivity to dFdC in MCL, and a good correlation was found between hENT-1 expression and uptake and sensitivity to this nucleoside analog in previous studies.39 Similarly, RRM2 overexpression has been reported as a mechanism of resistance to dFdC in cell lines,13 and overexpression was correlated with poor survival in lung adenocarcinoma patients treated with docetaxel and dFdC.47 We observed a synergistic effect between MI-63 and dFdC in some of the MCL patient samples, whereas others were resistant to this combination. The resistance may be linked to other cellular factors beyond those we evaluated here, because dFdC can be transported, metabolized, and inactivated by additional factors, including the human equilibrative nucleoside transporter-2, the human concentrative nucleoside transporters-1 and -2, cytidine deaminase, and 5′-nucleotidase, among others. For example, 1 MCL patient who was resistant to nucleoside analogs demonstrated abundant hENT-1, dCK, and minimal 5′-nucleotidases, but it was still resistant on presentation to fludarabine and dFdC.48 Moreover, alterations in genes such as ataxia telangiectasia mutated and p53 may influence resistance to nucleoside analogues,49 particularly in MCL given the high rate of changes in DNA damage pathways.21,22 It is also likely that the prior chemotherapy regimens patients received will have had an effect on the sensitivity to the MI-63–dFdC combination, particularly in those who received regimens incorporating DNA-damaging agents.
The cis-imidazoline HDM-2 inhibitor RO5045337 is currently undergoing phase 1 trials targeting patients both with solid tumors (NCT00559533) and with hematologic malignancies such as chronic lymphocytic leukemia (NCT00623870). With regard to the latter study, a recent preliminary report indicated that 47 patients had been treated to date, and pharmacodynamic studies had shown induction of p53 target genes such as p21, Bax, and Fas.50 Dose escalation was continuing upward in 2 strata from 810 and 1920 mg/m2/day, respectively, and clinical activity was seen in 1 patient with acute myeloid leukemia at 360 mg/m2 who had achieved an ongoing complete remission for > 9 months. These preliminary results of the first clinical trial of an HDM-2 inhibitor provide a proof of principle that the p53–HDM-2 axis can be manipulated with specific inhibitors not only preclinically but also clinically. In combination with our current data, which provide a molecular rationale for combining an HDM-2 inhibitor and dFdC in models of MCL, especially if they have wt p53 and express hENT-1 and dCK, these findings provide a strong framework for translation of this approach to the clinic. Moreover, because nucleoside analogs are used in other subtypes of non-Hodgkin lymphomas, as well as other hematologic malignancies, including acute and chronic leukemias, both in the relapsed and in some front-line settings, investigation of this regimen may be warranted preclinically and clinically in those settings as well.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Primary tumor samples were provided by the M. D. Anderson Cancer Center Lymphoma Tissue Bank (P50 CA136411). Flow cytometry services were provided by the M. D. Anderson Flow Cytometry Core Facility, and p53 gene sequencing was performed by the DNA Analysis Core Facility, all of which are supported by Cancer Center Support grant CA 16672. The authors are grateful to Shaomeng Wang (University of Michigan) for providing MI-63 and MI-219 and to Lance Leopold (Ascenta Therapeutics) for valuable suggestions.
R.J.J., a Lymphoma Research Foundation Fellow, acknowledges support from the Lymphoma Research Foundation. R.Z.O., a Leukemia & Lymphoma Society Scholar in Clinical Research, acknowledges support from the National Cancer Institute (grant P50 CA142509).
National Institutes of Health
Authorship
Contribution: R.J.J. designed and performed all of the research and animal experiments and wrote the manuscript; L.E.F., J.E.R., and M.W. obtained patient consent and samples; R.S. and S.N. were essential for helping with processing of patient samples and maintaining the lymphoma tissue bank; D.Y. provided the MI-63 and MI-219 and method; V.B. performed statistical support and analysis; and R.Z.O. supervised all the research completed here and offered valuable suggestions and manuscript editing.
Conflict-of-interest disclosure: D.Y. is an employee of and stockholder in Ascenta Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Richard J. Jones, Department of Lymphoma and Myeloma, The University of Texas M. D. Anderson Cancer Center, 7455 Fannin St, Unit 403, Houston, TX 77054; e-mail: rjones@mdanderson.org.