Abstract
The Akt family of serine/threonine kinases includes Akt1, Akt2, and Akt3 isoforms. Prior studies have reported that Akt1 and Akt2, but not Akt3, are expressed in platelets. Here, we show that Akt3 is expressed in substantial amounts in platelets. Akt3−/− mouse platelets selectively exhibit impaired platelet aggregation and secretion in response to low concentrations of thrombin receptor agonists and thromboxane A2 (TXA2), but not collagen or VWF. In contrast, platelets from Akt1−/− or Akt2−/− mice are defective in platelet activation induced by thrombin, TXA2, and VWF, but only Akt1−/− platelets show significant defects in response to collagen, indicating differences among Akt isoforms. Akt3−/− platelets exhibit a significant reduction in thrombin-induced phosphorylation of glycogen synthase kinase 3β (GSK-3β) at Ser9, which is known to inhibit GSK-3β function. Thus, Akt3 is important in inhibiting GSK-3β. Accordingly, treatment of Akt3−/− platelets with a GSK-3β inhibitor rescued the defect of Akt3−/− platelets in thrombin-induced aggregation, suggesting that negatively regulating GSK-3β may be a mechanism by which Akt3 promotes platelet activation. Importantly, Akt3−/− mice showed retardation in FeCl3-induced carotid artery thrombosis in vivo. Thus, Akt3 plays an important and distinct role in platelet activation and in thrombosis.
Introduction
Platelets are critical for hemostasis, but under pathologic conditions, are also important in thrombosis.1 Platelet activation is initiated at sites of vascular injury on exposure to soluble agonists such as thrombin, ADP, and thromboxane A2, and adhesion to subendothelial matrix proteins, such as von Willebrand factor and collagen.2 These adhesive proteins and agonists stimulate an intracellular signal transduction cascade leading to transformation of the major platelet adhesion receptor, integrin αIIbβ3 from its resting to active state (inside-out signaling), which allows the integrin to bind fibrinogen, and therefore mediate platelet aggregation.3,4 Activated platelets secrete proaggregating factors and adhesive glycoproteins from granules, which further cause stabilization and amplification of aggregation, leading to thrombus formation. Ligand binding to the activated integrin αIIbβ3 also transmits “outside-in” signals, which are critically important in stable platelet adhesion, spreading, and clot retraction.3,5,6
Elucidation of the signaling pathways regulating platelet activation is essential for the identification of novel anti-thrombotic targets for the prevention of thrombosis, a major cause of heart attack and stroke. It is established that phosphoinositide 3-kinases (PI3K) play important roles in platelet activation.7-12 Akt (also known as Protein Kinase B or PKB), the most well known effector of PI3K, is activated downstream of PI3K during platelet activation.9,13,14 Akt is a family of serine/threonine kinases with 3 isoforms: Akt1, Akt2, and Akt3 (for reviews see Manning et al15 and Bhaskar et al16 ). Akt isoforms are 80% homologous in their protein sequences. However, knockout mouse models of Akt isoforms have revealed distinct phenotypes, suggesting the possibility of unique functional roles of Akt isoforms or differences in expression levels of Akt isoforms in specific tissues.17-19
It has been demonstrated that platelets express Akt1 and Akt2.20 Knockout of Akt1 or Akt2 in mice results in similar defects in platelet activation induced by thrombin, VWF, and TXA2, but only Akt1−/− platelets showed significant defects in collagen-induced platelet activation. These observations suggest that Akt1and Akt2 are both important in platelet activation, but may have different roles.21-24 It has been reported that Akt3 was not detectable in platelets.20 However, in our study, we have obtained evidence that Akt3 is not only present in platelets, but also is a major Akt isoform expressed both in human and mouse platelets. Akt3 knockout mouse platelets selectively exhibit impaired platelet aggregation and secretion in response to stimulation by thrombin and TXA2 receptors, but not collagen or VWF, which is different from either Akt1 or Akt2. Importantly, Akt3 knockout mice exhibit impaired thrombus formation in vivo and in vitro compared with wild-type mice. Thus, Akt3 plays an important and distinct role in platelet activation and thrombosis.
Methods
Animals
The generation of Akt3 knockout mice has been previously described.19 Akt3−/− mice are on a mixed 129R1/C57BL background. Wild-type control mice and Akt3−/− mice used in this study were 8-15 week-old littermates generated from heterozygous breeding. Animal usage and protocol were approved by the institutional animal care committee of the University of Illinois at Chicago.
Preparation of platelets
For studies using human platelets, fresh blood was drawn by venipuncture from healthy volunteers and anti-coagulated with one-seventh volume of acid-citrate dextrose (ACD) as previously described.9 Institutional Review Board approval was obtained from the University of Illinois at Chicago, and informed consent was provided according to the Declaration of Helsinki. For the preparation of mouse platelets, fresh blood was drawn from mouse inferior vena cava and anti-coagulated with ACD as previously described.25 Blood from 5-6 mice of same genotype was pooled and platelets were isolated by differential centrifugation of whole blood with 0.1 μg/mL prostaglandin E1 and 1 U/mL apyrase (Sigma-Aldrich). Platelets were washed twice with CGS buffer (sodium chloride 0.12M, trisodium citrate 0.0129M, D-glucose 0.03M; pH 6.5), resuspended in modified Tyrode's buffer and allowed to rest for at least 1 hour at room temperature before use.26 For some experiments, platelets were washed and resuspended according to Liu et al.27
RT-PCR from platelet cDNA
RNA was isolated from human, Akt3+/+ or Akt3−/− mouse platelets (3 × 108) using Trizol Reagent (Invitrogen). Total RNA was reverse transcribed using Thermoscript RT-PCR kit (Invitrogen). Akt3 cDNA was amplified over 35 cycles with a forward 5′ATG AAT TGT AGC CCA GCC TCA CAG ATT3′ and reverse 5′CAT GCC GTC GTC GTC ATA CTT TTC3′ primer for mouse Akt3; and with a forward 5′GAT GCC TCT ACA ACC CAT CAT3′ and reverse 5′GTC CAT GCA GTC CAT ACC ATC CT3′ for human Akt3. PCR products were separated on a 1% agarose gel containing ethidium bromide and visualized under UV lamp. Control PCR reaction was performed using the same cDNA preparations using primers specific for GAPDH. To exclude the possibility of contamination from leukocytes, RNA template was isolated from washed leukocytes and RT-PCR was performed using identical primers and PCR conditions as described for platelets. Isolation of leukocytes was performed as previously described.28
Immunoabsorption
Washed human platelets (1 × 109/mL) resuspended in Tyrode's buffer were solubilized with an equal amount of solubilization buffer 1% Triton X-100, 150mM NaCl, 50mM Tris, containing 10mM EGTA, 0.2mM E64, 1 mM phenylmethylsulfonyl fluoride, and 1 unit/mL aprotinin and incubated on ice for 20 minutes. After centrifugation at 13 000g for 20 minutes at 4°C, the lysates (300 μL) were preincubated with anti-Akt3 antibody or control IgG overnight. After incubation for 1 hour with Protein A/G conjugated Sepharose beads (Santa Cruz Biotechnology), beads were separated from the lysates by centrifugation. This procedure was repeated once, and then the immunoabsorbed platelet lysates were analyzed by SDS-PAGE and immunoblotted with an anti-Akt3 and anti–total Akt antibody. Experiment was repeated 4 times. Quantitation was performed using National Institutes of Health (NIH) Image J Version 1.38X and paired t test was used for statistical analysis.
Immunoblot detection
Washed mouse platelets (3 × 108/mL) were incubated with or without thrombin in a platelet aggregometer at 37°C with stirring for various lengths of time. The reaction was stopped by addition of equal volume of sample buffer containing 2% SDS, 0.1M Tris, 2% glycerol, 2mM PMSF, 2mM Na3VO4, 2mM NaF, and Complete Protease Inhibitor Cocktail (Roche Molecular Biochemicals). Proteins were separated by SDS-PAGE on a 4%-15% polyacrylamide gel and then transferred to polyvinylidene difluoride membranes. The membranes were immunoblotted with an anti-Akt3 rabbit monoclonal antibody, anti–GSK-3β, anti–phosphoGSK-3β, anti-phosphoAkt Thr308, anti-phospho Akt Ser473, anti-Akt1, anti-Akt2 (Cell Signaling Technology), and total Akt (recognizing Akt1, Akt2 and Akt3; Santa Cruz Biotechnology Inc).
Platelet aggregation and secretion
Platelet aggregation and secretion was measured in a turbidometric platelet aggregometer (Chronolog) at 37°C with stirring (1000 rpm). Washed platelets (3 × 108/mL) in modified Tyrode's buffer were stimulated with thrombin (Enzyme Research Laboratories), collagen (Chronolog), U46619 (Calbiochem), and VWF and botrocetin (kindly provided by Dr Michael C. Berndt). Experiments were repeated at least 3 times. Platelet secretion was monitored in parallel with platelet aggregation as ATP release in a platelet lumiaggregometer (Chronolog) with the addition of luciferin/luciferase reagent (Chronolog) to the platelet suspension. Quantitation was performed using the ATP standard. To test the effects of GSK-3β inhibitor SB216763 (Sigma-Aldrich), SB216763 or DMSO was pre-incubated with platelets at 37°C for 2 minutes before addition of agonist. Aspirin (Sigma-Aldrich) was dissolved as 40mM stock solution in 0.2M HEPES, 0.15M NaCl, pH 7.8 (final pH 7.2) before added to platelets at 1mM final concentration.
Platelet adhesion under shear stress
Glass slides were coated with collagen (50 μg/mL) in water with acetic acid added to pH 3.0 overnight. Slides were washed with PBS and blocked with 5% BSA in PBS for 1 hour and washed again with PBS. Washed mouse platelets (200μL of 3 × 108/mL) were loaded onto the slides. A cone and plate rheometer (Rheostress 1, Thermo-HAAKE) was used to introduce shear stress (800 seconds−1) to the platelets. Mepacrine (10μM; Sigma-Aldrich), a fluourescent dye was added to the platelets before applying shear stress to the platelets for 5 minutes.23 Slides were rinsed in a container with 200 mL PBS 3 times to wash out nonstably adherent platelets. Slides were viewed with a Leica DMI RB fluorescence microscope (Leica Microsystems) using an N PLAN L lens at 40×/0.55 NA objective with 1.5× magnification.
In vivo thrombosis
Eight- to 10-week-old mice were anesthetized with isoflurane. The right carotid artery was isolated from surrounding tissues.25,29 A MA-0.5SB nanoprobe (Transonic Systems) was hooked to the artery and blood flow was monitored with a TS420 flowmeter (Transonic Systems). After stabilization, 1.2 μL of 5% FeCl3 (Sigma-Aldrich) was applied to a filter paper disc (2mM diameter) that was placed on top of the artery for 2 minutes. After removing the filter paper, blood flow was monitored continuously until 5 minutes after occlusion. Time to occlusion was calculated as a difference in time between the removal of the filter paper and stable occlusion (no blood flow for 2 minutes). Statistical analysis was performed using the Mann-Whitney test for the evaluation of differences in median occlusion time.
Results
Akt3 is a significant Akt isoform expressed in platelets.
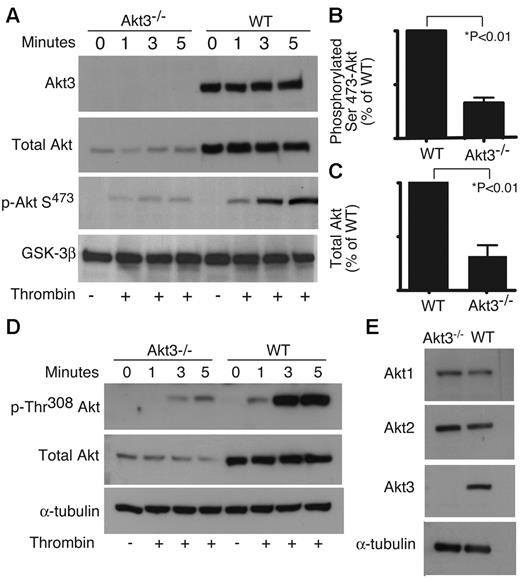
Important roles for Akt1 and Akt2 in platelets have been demonstrated by several groups.21-24,30 Akt3 was not detected in platelets in some previous studies.20 However, in our RT-PCR screen, a fragment of Akt3 mRNA was amplified using cDNA from purified platelets and oligonucleotide primers annealing to an Akt3-specific DNA sequence, and appeared to be abundant in human platelets (Figure 1A) and wild-type mouse platelets, but was not detected in Akt3−/− mouse platelets (Figure 1B). It is unlikely that the detected Akt3 mRNA was from contaminating leukocytes in the platelet preparation, because Akt3 was not detected when RNA from the same numbers of leukocytes as that contaminating platelet samples was used as a template (Figure 1B). Consistent with the expression of Akt3 mRNA in platelets, we also detected Akt3 protein in human and wild-type mouse platelets, but not in Akt3−/− platelets by Western blot with an Akt3-specific monoclonal antibody (Figure 1C). These data indicate that Akt3 is indeed expressed in human and mouse platelets.
Expression of Akt3 in platelets. (A) Human platelet RNA was isolated from washed platelets (3 × 108). RT-PCR was performed with primers specific for Akt3 or a housekeeping gene, GAPDH. (B) Mouse platelet RNA was isolated from 3 × 108 platelets of wild-type or Akt3−/− platelets and RT-PCR was performed similarly. Leukocyte contamination of platelet preparation was 4 × 104/mL as determined using Hemavet blood cell analyzer. RNA was isolated from 4 × 104/mL of WT mouse leukocytes and was also analyzed by RT-PCR using Akt3 specific primers under the same conditions as for platelet preparations to verify that the Akt3 fragment was not from leukocyte contamination. (C) Washed human platelets, wild-type and Akt3−/− mouse platelets were solubilized and immunoblotted with a rabbit antibody specifically recognizing Akt3, and α-tubulin is used as loading control. (D) Washed human platelets were solubilized, and immunoabsorbed with anti-Akt3 to remove Akt3 from lysates or with control rabbit IgG, and then immunoblotted with anti-Akt3 or an antibody recognizing all Akt isoforms (Total Akt). (E) Experiments in panel D were scanned and quantified using NIH Image J for uncalibrated optical density (mean ± SE, 4 experiments). The difference in percent of total Akt between IgG and Akt3 immunoabsorbed lysates is significant (P < .0125), as determined using paired t test.
Expression of Akt3 in platelets. (A) Human platelet RNA was isolated from washed platelets (3 × 108). RT-PCR was performed with primers specific for Akt3 or a housekeeping gene, GAPDH. (B) Mouse platelet RNA was isolated from 3 × 108 platelets of wild-type or Akt3−/− platelets and RT-PCR was performed similarly. Leukocyte contamination of platelet preparation was 4 × 104/mL as determined using Hemavet blood cell analyzer. RNA was isolated from 4 × 104/mL of WT mouse leukocytes and was also analyzed by RT-PCR using Akt3 specific primers under the same conditions as for platelet preparations to verify that the Akt3 fragment was not from leukocyte contamination. (C) Washed human platelets, wild-type and Akt3−/− mouse platelets were solubilized and immunoblotted with a rabbit antibody specifically recognizing Akt3, and α-tubulin is used as loading control. (D) Washed human platelets were solubilized, and immunoabsorbed with anti-Akt3 to remove Akt3 from lysates or with control rabbit IgG, and then immunoblotted with anti-Akt3 or an antibody recognizing all Akt isoforms (Total Akt). (E) Experiments in panel D were scanned and quantified using NIH Image J for uncalibrated optical density (mean ± SE, 4 experiments). The difference in percent of total Akt between IgG and Akt3 immunoabsorbed lysates is significant (P < .0125), as determined using paired t test.
To determine the relative amount of Akt3 expressed in human platelets, washed human platelets were solubilized and the lysates were immunoprecipitated with an anti-Akt3 monoclonal antibody to deplete the Akt3 protein (Figure 1D-E). As shown in Figure 2, this antibody is specific for Akt3 and does not cross react with Akt1 or Akt2, because no reactions were observed in Akt3−/− platelets, in which Akt1 and Akt2 are expressed normally. The levels of total Akt (all isoforms) or Akt3 that remained in platelet lysates were then determined by immunoblotting. Immunoabsorption with anti-Akt3 antibody, but not control IgG, depleted Akt3 from platelet lysates (Figure 1D), and resulted in an approximate 35% reduction in total Akt; therefore, Akt3 constitutes ∼ 35% of total Akt in human platelets (Figure 1E). To assess the relative amount of Akt3 present in mouse platelets, total Akt and phosphorylated Akt levels in wild-type and Akt3−/− platelets were measured by Western blot analysis using an antibody that recognizes all 3 isoforms and antibodies that recognize Thr308 and Ser473 phosphorylation sites of Akt, which are conserved among all Akt isoforms. Compared with wild-type platelets, Akt3−/− platelets exhibit a ∼ 70% reduction in total Akt (Figure 2A-B). In wild-type platelets, thrombin stimulates a time-dependent increase in phosphorylated Akt (at Thr308 and Ser473), which was also reduced by ∼ 70% in Akt3−/− platelets (Figure 2A,C,D). To exclude the possibility that Akt3 knockout caused dramatic changes in the expression levels of other Akt isoforms, we also analyzed Akt1 and Akt2 expression levels in wild-type and Akt3−/− mouse platelets using isoform-specific antibodies against Akt1 or Akt2. No significant difference in Akt1 or Akt2 expression was observed in Akt3−/− mouse platelets compared with wild-type (Figure 2E), indicating that the loss of total Akt protein observed in Akt3−/− mouse platelets is not because of loss of Akt1 or Akt2 proteins. Thus, our data indicates that Akt3 is expressed in a significant amount in platelets, and activated by platelet agonists.
Total and phosphorylated Akt in WT and Akt3−/− platelets. (A,D) Washed Akt3−/− and WT mouse platelets were stimulated with thrombin (0.018 U/mL) for 1, 3, and 5 minutes, solubilized, and immunoblotted with antibodies directed against: (A) Akt3, total Akt (Akt1, Akt2, and Akt3), phosphorylated Ser473 of Akt, and GSK-3β (loading control), and (D) phosphorylated Thr308 of Akt, total Akt and α-tubulin (loading control). (B,C) Western blot results from each of 3 experiments as shown in panel A were scanned and quantitated using NIH ImageJ for uncalibrated optical density. The relative quantity of total Akt (B) and phosphorylated Ser473 of Akt (C) in wild-type and Akt3−/− platelets are shown (mean ± SE). (E) Washed Akt3−/− and WT mouse platelets were solubilized and immunoblotted with antibodies directed against Akt1, Akt2, and Akt3 and α-tubulin (loading control).
Total and phosphorylated Akt in WT and Akt3−/− platelets. (A,D) Washed Akt3−/− and WT mouse platelets were stimulated with thrombin (0.018 U/mL) for 1, 3, and 5 minutes, solubilized, and immunoblotted with antibodies directed against: (A) Akt3, total Akt (Akt1, Akt2, and Akt3), phosphorylated Ser473 of Akt, and GSK-3β (loading control), and (D) phosphorylated Thr308 of Akt, total Akt and α-tubulin (loading control). (B,C) Western blot results from each of 3 experiments as shown in panel A were scanned and quantitated using NIH ImageJ for uncalibrated optical density. The relative quantity of total Akt (B) and phosphorylated Ser473 of Akt (C) in wild-type and Akt3−/− platelets are shown (mean ± SE). (E) Washed Akt3−/− and WT mouse platelets were solubilized and immunoblotted with antibodies directed against Akt1, Akt2, and Akt3 and α-tubulin (loading control).
The role of Akt3 in platelet secretion and aggregation
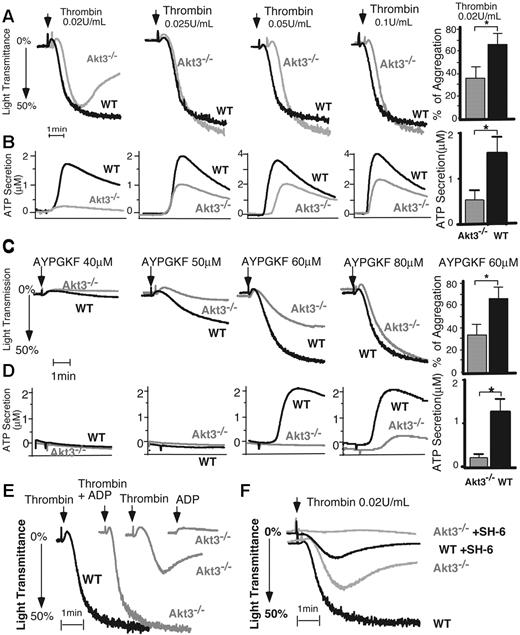
Akt3−/− mice were used to investigate the role of Akt3 in platelet activation. Akt3−/− mice exhibit no obvious abnormality in major hematologic parameters such as blood cell counts and hemoglobin levels. The size and counts of platelets in Akt3−/− mice are similar to that of wild-type mice. Akt3−/− platelets showed a partial reduction and reversal in platelet aggregation induced by a low dose of thrombin (Figure 3A), which is characteristic of a defect in platelet granule secretion. Platelet granule secretion is inhibited at this thrombin concentration (Figure 3B). Correspondingly, the defects in aggregation and secretion in Akt3 knockout platelets were also observed at low concentrations of PAR4 agonist peptide, AYPGKF (Figure 3C-D). At higher thrombin concentrations, the defect in platelet aggregation was overcome (Figure 3A). However, the granule secretion in Akt3−/− platelets is still reduced compared with wild-type platelets, indicating that the primary defect is in granule secretion (Figure 3B). Indeed, supplementing with a low concentration of granule content ADP (1μM), insufficient to induce aggregation on its own, reversed the inhibitory effect of Akt3 deficiency on thrombin-stimulated platelet aggregation (Figure 3E). Thus, Akt3 plays an important stimulatory role in mediating platelet secretion, and the secretion-dependent second wave of platelet aggregation induced by thrombin. Akt3−/− platelets also show a partial decrease in granule secretion induced by thromboxane A2 (TXA2) analog, U46619 (Figure 4A), and a delay in U46619-induced platelet aggregation (Figure 4A). However, the defects of Akt3−/− platelets in platelet secretion and aggregation induced by thrombin appear to be independent of TXA2 signaling pathway because Akt3−/− platelets showed decreased thrombin-induced platelet aggregation compared with wild-type platelets in the presence of a high concentration of aspirin. In addition, at the thrombin concentration in which aspirin reduced aggregation of wild-type platelets, Akt3−/− platelet aggregation was further reduced by aspirin (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The roles of Akt3 appear additive with that of Akt1/Akt2 because when SH-6, an isoform-nonselective inhibitor, is added to Akt3−/− platelets, platelet aggregation is further inhibited compared with Akt3−/− platelets alone (Figure 3F). Thus, different isoforms of Akt all play important roles in thrombin- and TXA2-induced platelet granule secretion and the secretion-dependent second wave of platelet aggregation. In fact, the defects of platelet aggregation stimulated with thrombin in Akt1−/−, Akt2−/−, and Akt3−/− platelets appear very similar in direct comparison (supplemental Figure 3A), all showing a defective second wave and stability of aggregation. However, Akt3−/− platelets do not show significant defects in platelet aggregation and secretion induced by collagen (Figure 4B), ADP (Figure 4C), and VWF/botrocetin (Figure 4D) even at very low concentrations. This selectivity is distinct from Akt1−/− platelets, which are defective in platelet activation induced by all these agonists,21-24 and also different from Akt2−/− platelets, which are defective in platelet activation induced by VWF, thrombin, and TXA2.22,23 Of the 3 isoforms, only Akt1 is required in platelet aggregation induced by low dose collagen, while neither Akt2 nor Akt3 knockouts showed significant effects on collagen-induced platelet activation (also see supplemental Figures 3B, 4B).22 Thus, our data indicate that Akt3 plays a stimulatory role selectively in the G protein-coupled thrombin receptor and the TXA2 receptor signaling pathways, which is distinct from Akt1 and Akt2. The difference in the roles of the 3 Akt isoforms in different platelet activation pathways suggests the different mechanisms of action of Akt1, Akt2, and Akt3 in stimulating platelet activation. Nevertheless, there may also be overlapping functions of different Akt isoforms.
Stimulatory role of Akt3 in platelet aggregation and secretion in response to thrombin and PAR4 agonist peptide. (A-D) Washed wild-type (WT) and Akt3−/− platelets were stimulated with thrombin (A,B) or PAR4 agonist peptide AYPGKF (C,D). Platelet aggregation was monitored using a turbidometric aggregometer at 37°C and 1000 rpm stirring speed (A,C). Platelet secretion of ATP was recorded concomitantly in the presence of luciferin-luciferase agent (B,D). Experiments described in panels A to D were repeated 3 times at a low dose of thrombin (0.02 U/mL) or AYPGKF (60μM) with the results quantified as percentage of light transmission (mean ± SE) or as concentration of secreted ATP (mean ± SE). (E) A low concentration of ADP (1μM), insufficient to induce aggregation on its own, reversed the inhibitory effect of Akt3−/− platelets on thrombin-stimulated aggregation. (F) Akt3−/− and WT mouse platelets were treated with vehicle DMSO or SH-6 (15μM) for 2 minutes and aggregation was recorded as shown in panel A.
Stimulatory role of Akt3 in platelet aggregation and secretion in response to thrombin and PAR4 agonist peptide. (A-D) Washed wild-type (WT) and Akt3−/− platelets were stimulated with thrombin (A,B) or PAR4 agonist peptide AYPGKF (C,D). Platelet aggregation was monitored using a turbidometric aggregometer at 37°C and 1000 rpm stirring speed (A,C). Platelet secretion of ATP was recorded concomitantly in the presence of luciferin-luciferase agent (B,D). Experiments described in panels A to D were repeated 3 times at a low dose of thrombin (0.02 U/mL) or AYPGKF (60μM) with the results quantified as percentage of light transmission (mean ± SE) or as concentration of secreted ATP (mean ± SE). (E) A low concentration of ADP (1μM), insufficient to induce aggregation on its own, reversed the inhibitory effect of Akt3−/− platelets on thrombin-stimulated aggregation. (F) Akt3−/− and WT mouse platelets were treated with vehicle DMSO or SH-6 (15μM) for 2 minutes and aggregation was recorded as shown in panel A.
Responses of Akt3−/− platelets to other platelet agonists. (A) Washed wild-type (WT) and Akt3−/− platelets were stimulated with U46619. Platelet aggregation was monitored using a turbidometric aggregometer at 37°C and 1000 rpm stirring speed. Platelet secretion was recorded concomitantly in the presence of luciferin-luciferase agent. Platelet aggregation was also measured after stimulation with (B) collagen (0.8 μg/mL, n = 6, P = .909), (C) ADP (for 2.5μM, n = 4, P = .1646) and (D) botrocetin and VWF (for Botrocetin 1.0 μg/mL, n = 3, P = .837). Quantitation was performed using Student t test.
Responses of Akt3−/− platelets to other platelet agonists. (A) Washed wild-type (WT) and Akt3−/− platelets were stimulated with U46619. Platelet aggregation was monitored using a turbidometric aggregometer at 37°C and 1000 rpm stirring speed. Platelet secretion was recorded concomitantly in the presence of luciferin-luciferase agent. Platelet aggregation was also measured after stimulation with (B) collagen (0.8 μg/mL, n = 6, P = .909), (C) ADP (for 2.5μM, n = 4, P = .1646) and (D) botrocetin and VWF (for Botrocetin 1.0 μg/mL, n = 3, P = .837). Quantitation was performed using Student t test.
The role of Akt3 in mediating GSK-3β phosphorylation
Akt has numerous substrates. We have previously shown that the role of Akt1 in stimulating platelet secretion and aggregation is mainly mediated by the NO/cGMP signaling pathway.24 Our data also suggest that the role of Akt2 in GPIb-IX–dependent platelet activation is also mainly mediated via the cGMP pathway.23 The roles of Akt1 in the NO/cGMP pathway is consistent with the data that Akt1−/− platelets showed defects in platelet granule secretion induced by nearly all tested platelet agonists,21,22,24 as cGMP is elevated by all of these platelet agonists.31,32 However, Akt3−/− platelets selectively showed a defect in thrombin- and TXA2-induced platelet secretion and aggregation, suggesting a possibly different downstream effector. It has been established that phosphorylation of GSK-3β at Ser9 by Akt negatively regulates GSK-3β function.33 Interestingly, previous studies showed that GSK-3β plays a negative regulatory role selectively in thrombin-induced platelet aggregation,34 but plays a stimulatory role in collagen-induced platelet aggregation.35 Thus, we investigated the possibility whether GSK-3β is a downstream effector of Akt3. Indeed, thrombin induced a significant increase in the phosphorylation levels of GSK-3β in wild-type platelets, which was partially, but significantly inhibited in Akt3 knockout platelets (Figure 5A-B). The role of Akt3 in phosphorylation of GSK-3β is not limited to the thrombin pathway. Phosphorylation of GSK-3β induced by other agonists such as collagen and ADP was also partially inhibited in Akt3−/− platelets (supplemental Figure 2). In contrast to the partial inhibition of GSK-3β phosphorylation in Akt3−/− platelets, treatment of platelets with the pan Akt inhibitor SH6 completely inhibited low-dose thrombin stimulated phosphorylation of GSK-3β at high SH6 concentrations (Figure 5C), suggesting the involvement of other Akt isoforms in addition to Akt3. Previously, it has been shown that platelets lacking 3 alleles of Akt (Akt1+/−Akt2−/−) exhibit a significant decrease in GSK-3β phosphorylation in PAR4 agonist peptide stimulated platelets compared with WT mouse platelets.34 Together, these data indicate that Akt3 plays an important role in mediating GSK-3β phosphorylation, but Akt isoforms other than Akt3 are also important in agonist-stimulated GSK-3β phosphorylation.
Phosphorylation of GSK-3β. (A) Washed Akt3−/− and WT mouse platelets were stimulated with thrombin (0.018 U/mL) for 1, 3, and 5 minutes, solubilized, and immunoblotted with antibodies against phosphorylated Ser9 of GSK-3β, and total GSK-3β (loading control). (B) Western blot results from each of 3 experiments as shown in panel A were scanned and quantified using NIH Image J for uncalibrated optical density. The relative quantity of phospho-GSK-3β in thrombin stimulated Akt3−/− versus that of wild-type mouse platelets is expressed as the percentage of wild-type (mean ± SE, 5 experiments). The difference in GSK-3β phosphorylation at resting, 1 minute, 3 minutes, and 5 minutes time points are significant between WT and Akt3−/− (P < .05), as determined using Student t test. (C) Washed WT mouse platelets were preincubated for 2 minutes with increasing doses of SH-6 or vehicle control DMSO and stimulated with thrombin (0.018 U/mL) for 3 minutes, solubilized, and immunoblotted with antibodies against phospho-Ser9 of GSK-3β, phospho-Ser473 of Akt, total Akt, and total GSK-3β (loading control).
Phosphorylation of GSK-3β. (A) Washed Akt3−/− and WT mouse platelets were stimulated with thrombin (0.018 U/mL) for 1, 3, and 5 minutes, solubilized, and immunoblotted with antibodies against phosphorylated Ser9 of GSK-3β, and total GSK-3β (loading control). (B) Western blot results from each of 3 experiments as shown in panel A were scanned and quantified using NIH Image J for uncalibrated optical density. The relative quantity of phospho-GSK-3β in thrombin stimulated Akt3−/− versus that of wild-type mouse platelets is expressed as the percentage of wild-type (mean ± SE, 5 experiments). The difference in GSK-3β phosphorylation at resting, 1 minute, 3 minutes, and 5 minutes time points are significant between WT and Akt3−/− (P < .05), as determined using Student t test. (C) Washed WT mouse platelets were preincubated for 2 minutes with increasing doses of SH-6 or vehicle control DMSO and stimulated with thrombin (0.018 U/mL) for 3 minutes, solubilized, and immunoblotted with antibodies against phospho-Ser9 of GSK-3β, phospho-Ser473 of Akt, total Akt, and total GSK-3β (loading control).
To further investigate whether the role of Akt3 in GSK-3β phosphorylation explains the selective role of Akt3 in thrombin-induced platelet aggregation, we tested the effects of a GSK-3β selective inhibitor, SB216763, on platelet aggregation induced by thrombin and collagen in wild-type and Akt3−/− platelets. Consistent with a previous report,34 SB216763 enhanced platelet aggregation and secretion induced by subthreshold concentrations of thrombin (Figure 6B). At higher concentrations of thrombin when platelet aggregation in normal platelets is already near maximal, there was no obvious effect of SB216763 on platelet aggregation (not shown). As expected, Akt3−/− platelets showed a decrease in platelet aggregation in response to low dose thrombin compared with wild-type platelets. In contrast, Akt3−/− platelets treated with SB216763 (10μM) totally rescued the defect of Akt3−/− platelets in thrombin-induced platelet aggregation (Figure 6A). Because we have shown that Akt3 is important in mediating phosphorylation of GSK-3β, which inhibits GSK-3β function, and that inhibition of GSK-3β function enhances thrombin-induced platelet aggregation, the rescue of the aggregation defect in Akt3−/− platelets by GSK-3β inhibitor suggests that Akt3-mediated regulation of GSK-3β function is likely to be sufficient for the role of Akt3 in promoting thrombin-induced platelet aggregation. In contrast to thrombin-induced platelet aggregation, collagen-induced platelet aggregation and secretion was significantly inhibited by SB216763 (Figure 6C), suggesting that GSK-3β does not promote platelet activation induced by the GPVI pathway. An inhibitory effect of SB216763 on platelet aggregation was also seen when ADP was used as an agonist (Figure 6D). These data suggest that GSK-3β positively regulates GPVI and ADP receptor signaling pathways. However, Akt3 knockout did not enhance platelet aggregation induced by collagen and ADP (Figure 4), despite of a role of Akt3 in GSK-3β phosphorylation (supplemental Figure 2), and SB216763 inhibited ADP-induced platelet aggregation in Akt3−/− platelets as in wild-type platelets (Figure 6D). These data suggest either that the partial phosphorylation of GSK-3β by other Akt isoforms are sufficient to mediate its regulation in collagen and ADP signaling pathways or an uncharacterized stimulatory role of Akt3 in these pathways masked the functional effect of Akt3 knockout on GSK-3β phosphorylation. Together, our data indicate that the selective role for Akt3 in promoting thrombin-induced platelet aggregation may be mainly mediated by the Akt3-dependent phosphorylation of GSK-3β and consequent inhibition of the negative regulatory signal of GSK-3β, although we do not exclude that other possible downstream effectors of Akt3 may also contribute to its role in platelet activation.
Reversal of the inhibitory effect of Akt3 knockout on platelet aggregation by GSK-3β inhibitor SB216763. (A) Washed WT and Akt3−/− platelets were pre-incubated for 2 minutes with 10μM of SB216763 or vehicle DMSO. These platelets were then stimulated with thrombin in a platelet aggregometer. (B) Washed WT mouse platelets were pre-incubated for 2 minutes with 10μM of SB216763 or vehicle DMSO and stimulated with a subthreshold concentration of thrombin in a platelet aggregometer. Platelet secretion was recorded concomitantly with aggregation in the presence of luciferase agent, and ATP release was recorded. (C) Washed mouse platelets were pre-incubated for 2 minutes with 10 μM of SB216763 or vehicle DMSO and then stimulated with collagen. Platelet ATP secretion was measured concomitantly. (D) Washed WT mouse platelets or Akt3−/− platelets were pre-incubated with 10μM of SB216763 or vehicle DMSO and then stimulated with ADP (5μM) in the presence of fibrinogen (10 μg/mL) and platelet aggregation was recorded.
Reversal of the inhibitory effect of Akt3 knockout on platelet aggregation by GSK-3β inhibitor SB216763. (A) Washed WT and Akt3−/− platelets were pre-incubated for 2 minutes with 10μM of SB216763 or vehicle DMSO. These platelets were then stimulated with thrombin in a platelet aggregometer. (B) Washed WT mouse platelets were pre-incubated for 2 minutes with 10μM of SB216763 or vehicle DMSO and stimulated with a subthreshold concentration of thrombin in a platelet aggregometer. Platelet secretion was recorded concomitantly with aggregation in the presence of luciferase agent, and ATP release was recorded. (C) Washed mouse platelets were pre-incubated for 2 minutes with 10 μM of SB216763 or vehicle DMSO and then stimulated with collagen. Platelet ATP secretion was measured concomitantly. (D) Washed WT mouse platelets or Akt3−/− platelets were pre-incubated with 10μM of SB216763 or vehicle DMSO and then stimulated with ADP (5μM) in the presence of fibrinogen (10 μg/mL) and platelet aggregation was recorded.
The role of Akt3 in platelet adhesion and thrombus formation under flow conditions in vitro
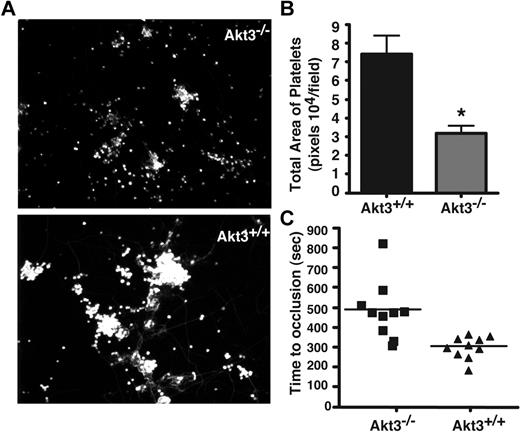
The important roles of different platelet activation pathways are to facilitate platelet adhesion and thrombus formation on exposed collagen surfaces of damaged blood vessel walls. To investigate whether the role of Akt3 in selective platelet activation pathways are relevant to thrombus formation on collagen surfaces under flow, we used a cone and plate rheometer to introduce flow with controlled shear rates to platelets on a collagen coated surface. A shear rate of 800 seconds−1 was used to mimic the physiologic shear rates of the arteries and arterioles. Washed platelets from both the wild-type or Akt3−/− mice stably adhered to the collagen-coated surface under this flow condition, suggesting that Akt3−/− platelets are not defective in adhesion to collagen. However, in contrast to wild-type platelets that formed large thrombi on the collagen coated surface, Akt3−/− platelets only formed smaller aggregates (Figures 7A-B), suggesting that Akt3−/− platelets are deficient in recruiting platelets into thrombi under high shear rate conditions.
Akt3 knockout delays formation of stable thrombi. (A) Washed mouse platelets (200 μL, 3 × 108/mL) were loaded onto slides coated with 50 μg/mL collagen and a cone and plate rheometer was used to introduce shear stress (800 seconds−1) to the platelets. Mepacrine, a fluourescent dye was added to the platelets before applying shear stress for 5 minutes. Slides were rinsed in a container with PBS to wash out nonstably adherent platelets. Slides were viewed with a Leica DMI RB fluorescence microscope (Leica Microsystems). (B) Quantitation of panel A using t test (P < .001). (C) FeCl3-induced carotid artery injury was performed, and time to occlusive thrombosis was recorded as described under “In vivo thrombosis.” The occlusion time of each mouse is shown as squares (Akt3−/−, n = 10) and triangles (Akt3+/+, n = 10). The bars represent the median occlusion time. Statistical analysis was performed using the Mann-Whitney test to evaluate the differences in median occlusion time (P = .0007).
Akt3 knockout delays formation of stable thrombi. (A) Washed mouse platelets (200 μL, 3 × 108/mL) were loaded onto slides coated with 50 μg/mL collagen and a cone and plate rheometer was used to introduce shear stress (800 seconds−1) to the platelets. Mepacrine, a fluourescent dye was added to the platelets before applying shear stress for 5 minutes. Slides were rinsed in a container with PBS to wash out nonstably adherent platelets. Slides were viewed with a Leica DMI RB fluorescence microscope (Leica Microsystems). (B) Quantitation of panel A using t test (P < .001). (C) FeCl3-induced carotid artery injury was performed, and time to occlusive thrombosis was recorded as described under “In vivo thrombosis.” The occlusion time of each mouse is shown as squares (Akt3−/−, n = 10) and triangles (Akt3+/+, n = 10). The bars represent the median occlusion time. Statistical analysis was performed using the Mann-Whitney test to evaluate the differences in median occlusion time (P = .0007).
Akt3 promotes in vivo thrombosis
To determine the in vivo physiologic relevance of the role of Akt3 in promoting platelet secretion and aggregation, we compared the in vivo thrombosis of wild-type and Akt3−/− mice using the FeCl3-induced carotid artery thrombosis model. The time to the formation of stable occlusive thrombus in the carotid artery is significantly prolonged in Akt3−/− mice compared with wild-type control mice (P = .0007, n = 10 for Akt3−/−, n = 10 for WT; Figure 7C). However, FeCl3-induced carotid artery thrombosis still occurred in Akt3−/− mice after a delay. This significant, but relatively mild anti-thrombotic effect of Akt3 knockout is consistent with its in vitro effect on thrombin and TXA2 induced platelet secretion and aggregation. These data suggest that Akt3 plays an important regulatory role in thrombus formation in vivo.
Discussion
In this study, we show that Akt3 is a significant Akt isoform expressed in platelets and plays an important role in platelet activation that is different from that of Akt1 and Akt2. We show that the role of Akt3 in platelets is likely mediated by phosphorylating and thus, negatively regulating GSK-3β. Furthermore, we demonstrate an important role for Akt3 in promoting stable thrombus formation in vivo.
It is well established that the PI3K signaling pathway has a critical function in platelet activation.7,9,10 The Akt family of protein kinases is an important downstream effector of PI3K signaling. Recent studies have shown that Akt1 and Akt2 play important stimulatory roles in platelet activation induced by low concentrations of platelet agonists.21-24 The role of Akt3 in platelets has never been studied, possibly because of a previous report that Akt3 was not detectable in platelets.20 However, our data clearly show that Akt3 is expressed in platelets in a substantial amount. Furthermore, our data indicate that Akt3−/− platelets have defects in thrombin (and TXA2)–induced platelet granule secretion and aggregation in vitro, and in thrombus formation in vivo, which are consistent with previous data that G protein-coupled thrombin receptor and TXA2 receptor pathways are important in thrombosis in vivo. Thus, we conclude that Akt3 plays important roles in platelet function and thrombosis. Together with previous findings of important roles of Akt1 and Akt2 in platelets, and additive roles of Akt3 and other Akt isoforms, our data further suggest that all 3 isoforms of Akt are important in promoting platelet granule secretion and thrombus formation, regardless of their relative quantities.
The role of Akt3 in promoting platelet activation appears to be selective for some of the platelet activation pathways, but is not involved in a general signaling pathway. Akt3−/− platelets showed significant defects in platelet activation induced by thrombin receptor agonists and (to a lesser degree) TXA2-mediated platelet secretion and aggregation, but had no defect in platelet secretion and aggregation induced by collagen, ADP and VWF/botrocetin. The defect of Akt3−/− platelets selectively in certain platelet activation pathways is clearly different from Akt1−/− platelets, which have been shown to be defective in platelet secretion and aggregation induced by all tested agonists including VWF, collagen, and ADP,21-24 and is also different from Akt2−/− platelets which is involved in VWF/GPIb-mediated platelet activation, in addition to thrombin and TXA2 pathway. Thus, the 3 isoforms of Akt play different roles in different platelet activation signaling pathways. Most of our current knowledge on the roles of Akt family of kinases in physiology and pathology were obtained with the prototype Akt isoform, Akt1 and to a degree, Akt2. Akt3 has been the least characterized, perhaps because Akt3 was thought to be a redundant Akt isoform. The functional redundancy between different Akt isoforms is evident, because more dramatic defects and early death only occur in mice with the genes of at least 2 Akt isoforms deleted. However, deletion of different individual Akt isoforms results in different phenotypes, suggesting that different Akt isoforms may also play distinct roles.19 Although, it is also possible that the distinct phenotype results from different tissue distribution patterns or localization of Akt isoforms. Our studies provide novel evidence of distinct roles of different Akt isoforms in the same cell.
Akt family of protein kinases has many substrates, and plays multiple roles in many aspects of life and in many types of cells. For example, Akt1 has been shown to phosphorylate and activate endothelial nitric oxide synthase (eNOS),36 and thus stimulate nitric oxide (NO)-cGMP signaling pathway in many types of cells including platelets.24 We have shown that Akt1 is important in platelet cGMP elevation induced by all tested agonists, and promotes platelet activation, particularly granule secretion, via the NO/cGMP pathway.23,24 Akt2 has been shown to be important in GPIb-IX-mediated cGMP elevation. However, how Akt2 plays a role in platelet activation remains unclear. The data presented in this study suggest that Akt3 mediates platelet activation mainly by negative regulation of another Akt substrate, GSK-3β.
It has been established that phosphorylation of GSK-3β at Ser9 by Akt family of kinases negatively regulates GSK-3β function.33 The role of GSK-3β in platelet activation has been examined by several groups. Some studies showed that pharmacologic inhibition of GSK-3β reduced platelet aggregation induced by collagen,35 but another group showed that both pharmacologic GSK-3β inhibitors and partial reduction of GSK-3β in heterozygous GSK-3β knockout platelets enhanced platelet aggregation induced by thrombin receptor agonists.34 In our studies, GSK-3β selective inhibitor SB216763 inhibited collagen- and ADP-induced platelet activation, but enhanced platelet aggregation induced by a subthreshold concentration of thrombin, suggesting that GSK-3β plays differential roles in different platelet activation pathways, which explains previous controversies. The differential roles of GSK-3β in different platelet activation pathways are consistent with the selective role of Akt3 in different platelet activation pathways. Indeed, we observed a significant reduction in GSK-3β phosphorylation in Akt3−/− platelets stimulated with thrombin, indicating that Akt3 is an important kinase responsible for GSK-3β Ser9 phosphorylation. Furthermore, treatment of Akt3−/− platelets with GSK-3β inhibitor SB216763 completely corrected the defect in thrombin-induced platelet aggregation in Akt3−/− platelets. These data suggest that Akt3 is likely to play a stimulatory role in low dose thrombin-induced platelet activation by inactivating the negative regulatory role of GSK-3β. It is important to note that GSK-3β is regulated not only by Akt3, but also by other Akt isoforms, which may also contribute to its function. However, clearly different functional phenotypes between 3 different Akt isoforms suggest that other downstream effectors, such as the NO/cGMP pathway, may be important in the functional effects of these Akt isoforms. In addition, we do not exclude the possibility that Akt3 may also regulate other unidentified substrates, which is interesting for further study. The mechanism by which GSK-3β exerts its effects is unclear at this stage. A recent study suggests a role for GSK-3β in the Wnt signaling pathway in platelets.37 Thus, more detailed studies to determine the roles of GSK-3β on platelet function downstream from Akt isoforms are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from NHLBI/NIH, HL068819, HL062350, and HL080264 (X.D.); and from CA090764, AG016927, and AG025953 (N.H.).
National Institutes of Health
Authorship
Contribution: K.A.O. performed a major part of experiments, analyzed data and wrote the paper; A.S.-T. performed a part of the experiments and data analysis; N.H. was involved in research design, data analysis, discussions and provided Akt3−/− mice; and X.D. designed the research, analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaoping Du, Department of Pharmacology, University of Illinois College of Medicine, 835 S Wolcott Ave, Chicago, IL 60612; e-mail: xdu@uic.edu.