Abstract
The main objective of this study was to investigate whether patients with chronic myeloid leukemia (CML) in treatment with long-term therapy imatinib have a different health-related quality-of-life (HRQOL) profile compared with the general population. In total, 448 CML patients were enrolled, and the SF-36 Health Survey was used to compare generic HRQOL profiles. Symptoms were also assessed. HRQOL comparisons were adjusted for key possible confounders. The median age of patients was 57 years and the median time of imatinib treatment was 5 years (range 3-9 years). The largest HRQOL differences were found in younger patients. In particular, patients aged between 18 and 39 years had marked impairments in role limitations because of physical and emotional problems, respectively: −22.6 (P < .001), −22.3 (P < .001). Patients with CML age 60 or older had a HRQOL profile very similar to that reported by the general population. Women had a worse profile than men when each were compared with their peers in the general population. Fatigue was the most frequently reported symptom. The HRQOL of CML patients is comparable with that of population norms in many areas, however, younger and female patients seem to report the major limitations.
Introduction
The treatment of chronic myeloid leukemia (CML) has changed dramatically with the advent of targeted therapies.1 Results from the landmark International Randomized Study of Interferon and STI571 (IRIS) comparing IFNα plus low-dose cytarabine (LDAC) with imatinib for the clinical management of CML patients led to the adoption of the first targeted therapy (ie, imatinib) as standard first-line treatment.2 Overall survival at 8 years is 85%; for only CML-related deaths and those before stem cell transplantations, the survival is 93%.3 The IRIS trial also showed that imatinib provided major advantages in terms of health-related quality of life (HRQOL) over IFNα + LDAC. The effect size favoring the imatinib arm was shown to be one of the largest ever reported in the HRQOL-randomized controlled trial–based literature.4 Although HRQOL advantages were found in this pivotal analysis of the IRIS study, the follow-up was up to 1 year. Another study has been recently published documenting the HRQOL impact of imatinib but patients were only followed up from diagnosis to 6 months.5
A recent systematic review on patient-reported outcomes (PROs) in CML patients has shown that no full data exist on the long-term burden of the disease and treatment-related side effects from the patients' perspective.6 The identification of subgroups of CML patients who are at higher risk for late consequences of treatment in some specific HRQOL areas could guide the development of more tailored supportive care programs. Clinicians are now confronted with new challenges in patient management and ensuring the highest possible long-term HRQOL outcomes are among the top priorities of the current CML research agenda. As pointed out by Pinilla-Ibarz and colleagues, data on the effects of imatinib on HRQOL could also contribute to further defining the concept of intolerance in CML patients receiving long-term chronic therapy as the traditional use of the Common Toxicity Criteria might be limited in this context.7
The main objective of this study was to investigate to what extent patients with CML receiving long-term therapy with imatinib have a different HRQOL profile compared with a cohort of matched control subjects from the general population. A secondary objective was to describe symptom prevalence from the patients' perspective.
Methods
Study design and study population
Between March and December 2009, CML patients were enrolled in a multicenter cross-sectional study including 26 centers. These centers covered 15 of the 20 regions of Italy and were balanced by the 3 main geographic areas (northern, central, and southern) of the country. Eligibility criteria included: adult patients with CML who started imatinib as first-line therapy in the early chronic phase of the disease and receiving imatinib treatment for at least 3 years. Patients had to be at least in complete cytogenetic response (CCyR) at the time of study entry. Patients with a secondary malignancy or those who had received any type of previous treatment were not eligible. The study was approved by the ethic committees of each participating center and all patients provided written informed consent in accordance with the Declaration of Helsinki.
Study procedures and data collection
Patients were identified through hospital medical records and were invited to participate by their own treating physician in the hospital. The protocol specified that all eligible patients were to be approached and invited to participate at the earliest convenience. To ensure a uniform recruitment process, a national investigator meeting including representatives from all participating centers was set up before the start of the study to discuss the logistics and to provide written study-specific standard operating procedures. Investigators had to inform patients that possible nonparticipation in this study would not have any consequence on their follow-up care. All eligible patients were informed about the purpose of this study and those who consented to participate were given a survey booklet along with a self-addressed stamped envelope. Patients were requested to complete the survey at home at their earliest convenience. All surveys were returned to an independent national data center. Investigators provided information on the full medical history of the patients, which was linked to the returned surveys to build up the study database.
Patient-reported outcome data collection
Comparisons with the general population norms were performed with the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36, Version 1), the most well-established generic HRQOL measure used in survivor populations.8,9 The SF-36 measure has been validated for the Italian population and has been shown to be of value in monitoring HRQOL in long-term cancer survivors.10,11 The questionnaire consists of 36 items yielding 8 domains: physical functioning (PF), role limitations because of physical problems (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitations because of emotional problems (RE), and mental health (MH). The 8 domains have a score ranging between 0 and 100 with higher scores representing better health outcomes. Two summary scores, namely the physical component summary (PCS) and the mental component summary (MCS), are derived from a weighted combination of the 8 scales. PF, RP, BP, and GH have the higher weights for the PCS whereas VT, SF, RE, and MH have higher weights for the MCS. All scores on the SF-36 of our CML population were compared against previously published Italian population norms10,12 and adjusted for the following key possible HRQOL confounders: age, sex, education, geographic area, and marital status.13-15 Patient reported symptoms were measured with an ad hoc 9-item checklist for CML patients undergoing imatinib treatment.16 Items were rated on a 4-point Likert scale to be consistent with validated symptom scales/items included in other psychometric robust measures.17 The following symptoms were evaluated: abdominal discomfort, diarrhea, edema, fatigue, headache, muscle cramps, musculoskeletal pain, nausea, and skin problems.
Statistical methods
SF-36 domain and summary scores were calculated according to standard scoring guidelines.12,18 Differences between groups were assessed using the Fisher exact test, and Wilcoxon-Mann-Whitney and Kruskall-Wallis tests as appropriate. To minimize possible bias comparing HRQOL outcomes of CML patients to national population norms, controls from disaggregated normative data were matched to CML subjects by propensity score matching.19 Similar approaches have been used in previous HRQOL studies.20 The prematching control group consisted of a representative sample of 1997 Italian adults without cancer from a previous nationwide study aimed at validating and providing population norms for the SF-36.10 We implemented an optimal matching procedure by selecting the best possible case-control pairs given the available data. Matching was based on age, sex, education, and geographic area. Because of matching procedures, all comparisons between CML patients and matched population controls were based on 409 patients per group. Adjusted mean differences were determined with multiple regression, using selected a priori key HRQOL confounders as covariates: age, sex, education, geographic area, and marital status. The choice of age categories for descriptive comparisons between CML patients and general population norms was based on previous similar population-based studies.21 Statistical tests for significance of adjusted mean differences of the 8 SF-36 scales were Bonferroni corrected for multiple comparisons. Eight points were considered to be a minimally important difference (MID) for the first 8 SF-36 scales,22 while a difference of 2 points was judged as MID for the PCS and the MCS scores.23,24 A score difference at least equal to MID was considered as a clinically meaningful difference. For descriptive purposes, standardized effect sizes (z-scores) were graphically presented for the 8 SF-36 scales as well as for the 2 summary scores. For the 8 SF-36 scales, each group-specific adjusted mean difference was standardized by the corresponding SD in the matched control group. PCS and MCS were computed for CML and the matched control group using general population mean scores. The frequency distribution of response categories for symptoms was calculated and each symptom was reported as “severe” if the response was at least “quite a bit.”25 All analyses were performed with SAS Version 9.1.3 (SAS Institute Inc).
Results
In total of the 472 eligible patients approached during the study period, 448 patients were registered in this study. Twenty-two patients refused to participate as they were not interested in the purpose of the study and a further 2 were not enrolled for logistical reasons. Of those registered, 422 (94%) returned the survey booklet (ie, respondents). A comparison of respondents versus nonrespondents revealed no statistically significant differences for sociodemographic and clinical or treatment-related data except for performance status. Details are reported in Table 1.
Overall CML population compared with population norms
Clinically meaningful differences were observed for RP (−11.5; 95% confidence interval [CI], −16.8 to −6.3), GH (−8.9; 95% CI, −11.7 to −6.0), and RE (−9.6; 95% CI, −14.9 to −4.3) scales. CML patients had a clinically significant worse PCS score than population controls (−2.4; 95% CI, −3.6 to −1.3) but not for the MCS (−0.9; 95% CI, −2.3 to 0.4). Mean scores and adjusted mean differences for the SF-36 scales for CML population compared with control subjects are presented in Table 2.
Age group comparisons with population norms
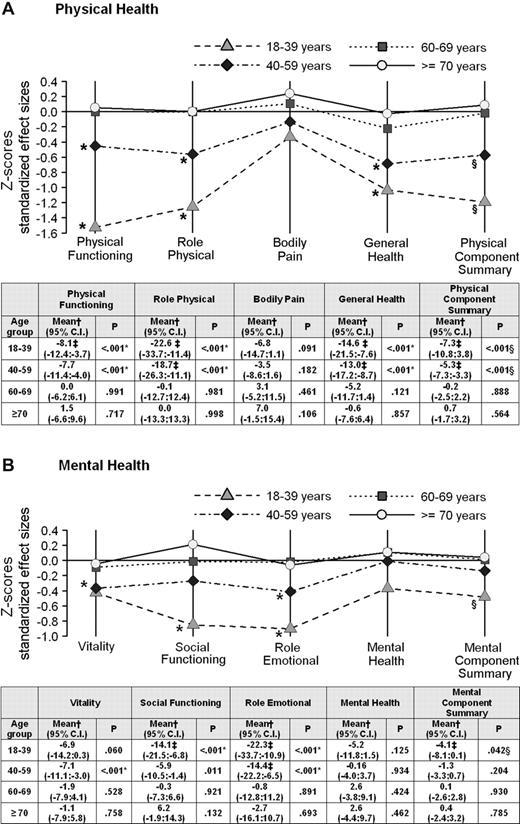
Worse clinically significant scores were observed for the 2 younger groups (age 18-39 and 40-59 years) in several scales. In patients between 18 and 39 years of age, adjusted mean differences with the control group were more than twice the magnitude of a clinically meaningful difference for the RP (−22.6; 95% CI, −33.7 to −11.4) and the RE (−22.3; 95% CI −33.7 to −10.9) scales. Patients in this age group also reported clinically worse outcomes compared with the control group for the GH, SF, and PF scales. In the 40- to 59-year age group there were major limitations for the RP, RE, and GH scales in the CML group. Comparisons between CML patients between 60 and 69 years of age and patients 70 years and older with population norms showed almost identical scores in all scales (Figure 1). Additional analyses showed that there were no significant differences in terms of duration of therapy and time since diagnosis for all CML age group categories.
Adjusted mean differences between CML patients and their respective control groups by age categories. (A) Physical health and (B) mental health. A score below 0 line means worse outcomes for CML patients. Note that connecting lines among SF-36 outcomes are plotted only for descriptive purposes. *Statistically significant after Bonferroni adjustment (adjusted α = 0.05/8 = 0.00625). Statistical significance refers to the group specific adjusted mean differences of SF-36 scores between CML patients and matched control subjects. †Mean difference between CML patients by age group and the respective matched control subjects adjusted for age, gender, education, geographical area, and marital status. A negative sign indicates worse outcomes for CML patients. ‡Exceeds minimally important difference (ie, 8 points for the SF-36 scales and 2 points for the PCS and MCS scores). §Statistically significant (α = 0.05).
Adjusted mean differences between CML patients and their respective control groups by age categories. (A) Physical health and (B) mental health. A score below 0 line means worse outcomes for CML patients. Note that connecting lines among SF-36 outcomes are plotted only for descriptive purposes. *Statistically significant after Bonferroni adjustment (adjusted α = 0.05/8 = 0.00625). Statistical significance refers to the group specific adjusted mean differences of SF-36 scores between CML patients and matched control subjects. †Mean difference between CML patients by age group and the respective matched control subjects adjusted for age, gender, education, geographical area, and marital status. A negative sign indicates worse outcomes for CML patients. ‡Exceeds minimally important difference (ie, 8 points for the SF-36 scales and 2 points for the PCS and MCS scores). §Statistically significant (α = 0.05).
Sex comparisons with population norms
The HRQOL profile for female CML patients was generally worse than male CML patients. Marked impairments were found for female CML patients compared with population controls for RP (−17.9; 95%, CI, −26.8 to −9.1), RE (−13.8; 95%, CI, −22.5 to −5), and GH (-10.6; 95%, CI, −15.3 to −5.8) scales. Male CML patients showed no clinically meaningful differences with population controls for the 8 SF-36 scales (Figure 2). Additional analyses did not reveal any statistically significant differences by sex in terms of: time in treatment with imatinib, starting imatinib dose, ECOG performance status, Sokal risk classification, and comorbidity at diagnosis.
Adjusted mean differences between CML patients and their respective control groups by sex. (A) Physical health and (B) mental health. A score below 0 line means worse outcomes for CML patients. Note that connecting lines among SF-36 outcomes are plotted only for descriptive purposes. *Statistically significant after Bonferroni adjustment (adjusted α = 0.05/8= 0.00625). Statistical significance refers to the group specific adjusted mean differences of SF-36 scores between CML patients and matched control subjects. †Mean difference between CML patients by sex and the respective matched control subjects adjusted for age, education, geographical area, and marital status. A negative sign indicates worse outcomes for CML patients. ‡Exceeds minimally important difference (ie, 8 points for the SF-36 scales and 2 points for the PCS and MCS scores). §Statistically significant (α = 0.05).
Adjusted mean differences between CML patients and their respective control groups by sex. (A) Physical health and (B) mental health. A score below 0 line means worse outcomes for CML patients. Note that connecting lines among SF-36 outcomes are plotted only for descriptive purposes. *Statistically significant after Bonferroni adjustment (adjusted α = 0.05/8= 0.00625). Statistical significance refers to the group specific adjusted mean differences of SF-36 scores between CML patients and matched control subjects. †Mean difference between CML patients by sex and the respective matched control subjects adjusted for age, education, geographical area, and marital status. A negative sign indicates worse outcomes for CML patients. ‡Exceeds minimally important difference (ie, 8 points for the SF-36 scales and 2 points for the PCS and MCS scores). §Statistically significant (α = 0.05).
Patient-reported symptoms evaluation
Fatigue was the most prevalent symptom with 82% of patients reporting it with any level of concern. Inspection of the pattern of symptoms by sex broadly reflected the results of the HRQOL analysis. The 3 largest differences in terms of severe symptoms were found for edema: 16% versus 39%; fatigue: 22% versus 39%; and musculoskeletal pain: 18% versus 35%, respectively, for male and female. Exploratory multivariate analysis showed that sex effect on symptoms was independent of: age, education, duration of therapy, current dose of imatinib, Sokal risk at diagnosis, and comorbidity (data not shown). The overall frequency distribution of symptom severity for all symptoms is depicted in Figure 3.
Percentage of CML patients reporting the symptom by level of severity (N = 422).
Percentage of CML patients reporting the symptom by level of severity (N = 422).
Discussion
The main finding of this study was the pattern of HRQOL outcomes by different CML age group categories compared with their respective peers, without cancer, in the general population. Younger patients (18-39 years) had noticeable impairments in 5 of the 8 domains of the SF-36. In particular, major limitations in work or other daily activities because of physical and emotional problems (RP and RE scales of the SF-36) were evident. Marked impairments were also observed for physical and social functioning as well as overall health status and expectations for health in the future (PF, SF, and GH scales). This was also the only age group category reporting a significant worse MSC score. Clinically significant limitations for RP, GH, and RE scales were remained in patients between 40 and 59 years of age. Conversely, for patients 60 years or older, the HRQOL profile was very similar to their peers in the general population (Figure 1).
This finding is consistent with similar population-based studies conducted in other cancer diseases. Arndt and colleagues, for example, found that younger patients (18-59 years) with colorectal cancer reported major impact in terms of social, emotional, and role functioning compared with general population norms.21 One possible explanation may be that a long-term daily imatinib treatment schedule might have a greater impact in younger patients as they are more likely to have greater task demands in routine daily life. This could be supported by the evidence that only the youngest group (18-39 years) had major limitations in their self-reported evaluation of social functioning.
The only available evidence of HRQOL differences between CML patients and the general population is provided by Kiss and colleagues, who suggested that RP and GH were the most impaired scales of the SF-36.26 However, as this study investigated outcomes in a small cohort of subjects (N = 28) who received BM transplantation (BMT) no direct comparison with our results can be made. A comparison of our results with the broader literature on the long-term HRQOL effect of therapies in other cancer diseases is also difficult as the majority of prior investigations dealt with patients who were already off-treatment at the time of analysis.27,28
The magnitude of adjusted differences between female CML patients and their peers from the general population was larger than that of males with CML and their peers in all scales of the SF-36 (Figure 2). This finding might be consistent with some evidence from the IRIS trial showing that, independent of treatment effects, the average of HRQOL scores of men with CML was higher than that of women.4 Our results are also consistent with previous PRO data in CML patients undergoing BMT or IFN, which suggested worse outcomes for women in terms of psychosocial adjustment, anxiety, depression, and other symptoms.29-31 Analysis of symptom reporting in our CML sample showed a larger proportion of women reporting higher symptom severity. This is the first evidence suggesting a different pattern of HRQOL and symptom reporting by sex in CML patients receiving long-term therapy with imatinib.
Patient-reported symptom assessment showed that fatigue was the most frequently reported symptom and that between 25% and 30% of patients reported severe symptoms for edema, musculoskeletal pain, muscle cramps, and fatigue. As side effects of imatinib might be associated with lower adherence rates,32 it could be important to investigate the association of these long-term patient-reported symptoms with adherence to therapy. Further analyses are ongoing to investigate this issue within our study sample.
A strength of our study is the large sample size and the inclusion of CML patients not exclusively stemming from clinical trials, thus yielding a more realistic picture of routine practice. This article has also limitations. Because of the cross-sectional design, we were unable to control for HRQOL baseline values. Next, as we included patients undergoing imatinib for at least 3 years, our results can be generalized to only CML patients who were able to tolerate imatinib for so long. Another limitation was the use of an ad hoc symptom tool, as at present no fully internationally validated measure exists for CML patients.6,7 Nevertheless, this did not in anyway affect the primary objective of this study.
Our data add to the body of knowledge concerning long-term traditional clinical outcomes with imatinib treatment (eg, rates of CCyR and overall survival) by providing the patient's view on disease and treatment-related burden which is now highly valued by stakeholders.33
In conclusion, the HRQOL of patients with CML age 60 or older receiving long-term therapy with imatinib is comparable with that of their peers in the general population. However, particular attention should be focused on younger patients and women as they appear to report the largest differences compared with their peers in the general population. The results of this study will be of value to the physicians and patients to make more informed treatment decisions and will possibly contribute to raising the standards of survivorship care for this population.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in part at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December, 4-7, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to all patients who participated in this study, and acknowledge the essential contribution of the Associazione Italiana contro le Leucemie, Linfomi e Mieloma (AIL), which provided logistic and administrative support to this project. They are also grateful to Dr Paola Mosconi from the Istituto di Ricerche Farmacologiche Mario Negri (Milan, Italy) for her support in getting access to the SF-36 Italian validation data.
This study was supported by Novartis Italia. Novartis had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript.
Authorship
Contribution: F.E. was the study coordinator; M. Baccarani was the project's study co-coordinator; F.E., M. Baccarani, and F.M. were responsible for conception and design; F.E., M. Breccia, G.A., G.R., G.L.D., C.B., A.R.R., G.F., L. Luciano, D.T., B.M., F.D.R., M.D., M. Bergamaschi, P.L., M.P.S., L. Levato, S.U., D.V., S.S., and A.R. collected and assembled data; F.E., M. Baccarani, F.C., and F.M. interpreted and analyzed data; F.E. and F.C. provided statistical analysis; F.E., F.C., M.V., and F.M. wrote the manuscript; F.E., M. Baccarani, M. Breccia, G.A., G.R., F.C., G.L.D., C.B., A.R.R., G.F., L. Luciano, D.T., B.M., F.D.R., M.D., M. Bergamaschi, P.L., M.P.S., L. Levato., S.U., D.V., S.S., A.R., M.V., and F.M. gave final approval of the manuscript; and M. Baccarani, M. Breccia, G.A., G.R., G.L.D., C.B., A.R.R., G.F., L. Luciano, D.T., B.M., F.D.R., M.D., M. Bergamaschi, P.L., M.P.S., L. Levato, S.U., D.V., S.S., and A.R. provided study materials and patients.
Conflict-of-interest disclosure: F.E. served as a consultant or in an advisory role for Bristol-Myers Squibb; M. Baccarani, M. Breccia, G.R., F.D.R., and C.B. served as consultants or in advisory roles for Novartis and Bristol-Myers Squibb; F.E., G.R., F.D.R., and P.L. received research funding from Novartis; M. Baccarani received honoraria from Novartis, Bristol-Myers Squibb, Pfizer, and Ariad; G.A., G.R., A.R.R., and F.D.R. received honoraria from Novartis and Bristol-Myers Squibb; D.T. provided expert testimony for Novartis and Bristol-Myers Squibb; A.R. provided expert testimony for Novartis; and D.T. received other remuneration from Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
A complete listing of the GIMEMA membership can be found in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Fabio Efficace, PhD, Head, Health Outcomes Research Unit, Italian Group for Adult Hematologic Diseases (GIMEMA), GIMEMA Data Center, Via Benevento, 6, 00161-Rome, Italy; e-mail: f.efficace@gimema.it.