Abstract
Fcγ receptor (FcγR) polymorphisms have been shown to affect rituximab-mediated antibody-dependent cellular cytotoxicity. Of 512 patients with diffuse large B-cell lymphoma treated in the RICOVER-60 trial, carriers of FcγRIII 158 valine homozygous receptors (V/V) presented with a slightly decreased incidence of B-symptoms (158 V/V: 26%, V/F: 35%, phenylalanine receptors [F/F]: 42%; P = .037). Survival curves of all FcγR single nucleotide polymorphisms were superimposable after cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP); but after CHOP with rituximab (R-CHOP), event-free survival (EFS) and progression-free survival (PFS), but not overall survival, of FcγRIIIa 158 F/F had a trend to be lower than those of 158 V/F and 158 V/V: 3-year EFS: FcγRIIIa 158 F/F: 64.5%, 158 V/F: 70.2%, 158 V/V: 76.9% (log-rank test: P = .224 F/F vs V/V; P = .285 F/F vs V/F + V/V); 3-year PFS: FcγRIIIa 158 F/F: 68.3%, V/F: 76.1%, V/V: 80.5% (log-rank test: P = .233 for F/F vs V/V; P = .185 for F/F vs V/F + V/V). By multivariate analysis adjusting for International Prognostic Index factors, relative risk of F/F compared with V/F plus V/V was 1.80 (P = .052) for PFS and 1.55 (P = .120) for EFS. The interaction of R-CHOP, but not CHOP with FcγRIIIa polymorphisms, indicates a window of opportunity for CD20 antibodies designed to mediate enhanced antibody-dependent cellular cytotoxicity.
Introduction
Diffuse large B-cell lymphomas (DLBCLs) are the most common lymphoid neoplasms, accounting for ∼ 30% to 40% of all non-Hodgkin lymphomas. The introduction of rituximab (R) to the polychemotherapy combination of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)1 has significantly improved the outcome of all subgroups of patients.2-6 Mechanisms of action of rituximab include direct induction of apoptosis,7-9 chemosensitization of tumor cells to the cytotoxic effects of chemotherapy, complement-dependent cellular cytotoxicity,10-12 and antibody-dependent cellular cytotoxicity,11 of which the latter is thought to contribute most to the efficacy of this antibody against malignant cells of B-cell lymphomas.13,14 Antibody-dependent cellular cytotoxicity is mediated by effector cells that engage the Fc portions of the antibody via receptors for immunoglobulin (FcγRs). Three FcγR classes (FcγRI, FcγRII, and FcγRIII) and 8 subclasses have been described with significantly different haploptype distribution between various ethnic groups.15-17 FcγRIIIa (CD16a) is expressed on natural killer cells and macrophages, whereas FcγRIIa (CD32a) is expressed on neutrophils and macrophages. Genomic polymorphism of the FcγRIIIA corresponding to phenotypic expression of valine (V: guu/guc/gua/gug) or phenylalanine (F: uuc/uuu) at position 158 influences the binding of IgG1 to this receptor.18 It has been shown that natural killer cells with valine homozygous receptors (V/V) bind Fc better than those with phenylalanine receptors (F/F), resulting in more effective antibody-dependent cellular cytotoxicity.19 Patients with follicular lymphoma20,21 and Waldenström macroglobulinemia,22 but not with CLL23 carrying the FcγRIIIa 158 V/V phenotype, have been reported to respond better to rituximab monotherapy than F carriers, but this was not observed when rituximab was combined with CHOP.24-26 With respect to FcγRIIa, patients with follicular lymphoma and homozygous for histidine (H: cau/cuc) on position 131 were reported to respond better to rituximab monotherapy than patients heterozygous or homozygous for arginine (R: cgu/cgc/cga/cgg).21 However, this observation could not be confirmed by others.20,23,24,27 With respect to DLBCL, the response of 85 Korean patients treated with CHOP was the same among the carriers of different FcγRIIIa polymorphisms; but among 113 patients treated with R-CHOP, carriers of the FcγRIIIa 158 V/V were reported to respond better than F carriers to R-CHOP. However, this was not confirmed in a small series of 58 white patients.28 To evaluate, to the best of our knowledge, for the first time the role of FcγRIIa and FcγRIIIa polymorphisms on outcome of DLBCL patients who were treated uniformly within a prospective trial, where patients were randomly assigned to CHOP chemotherapy with and without rituximab, we examined the correlation of FcγRIIIa 158 V/F and FcγRIIa 131 H/R polymorphisms in patients treated within the RICOVER-60 study,4 with 1222 patients, the largest DLBCL study to date. In this trial, patients had been randomized into 4 arms: 6 and 8 cycles of biweekly CHOP (CHOP-14), each with and without 8 applications of rituximab.
Methods
This study was approved by the local ethics committee, the Ethikkommission der Ärztekammer des Saarlandes. The study was performed in accordance with the rules of the Declaration of Helsinki after obtaining written consent from the patients. Recombinant experiments were done with the permission and according to the rules of the government of Saarland.
Study population
The cohort consisted of 512 consecutive patients treated within the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (registered on National Cancer Institute website, no. CT0052936 and as EU-20243) from whom genomic DNA samples were available. All patients had untreated CD20+ aggressive B-cell lymphoma according to the World Health Organization classification29 as confirmed by reference pathology. Blood donors (n = 101) from the Institute for Transfusion Medicine, Saarland University Medical School served as controls.
DNA extraction and genotyping
Genomic DNA was isolated from whole blood with the QIAamp DNA Blood Kit (QIAGEN). DNA was diluted in water to a final concentration of 5 ng/μL to use 2.25 μL per reaction. The mutation tests were performed using the TaqMan (Invitrogen) SNP Genotyping Assays C_258156666_10 for the detection of the polymorphism rs396991 for the Fc-γ receptor IIIa (CD16a) and C_9077561_20 for the detection of the polymorphism rs1801274 of the low-affinity Fc-γ-IIa (CD32a) receptor, respectively. A total of 2.25 μL genomic DNA was mixed with 2.5 μL TaqMan Universal Maser Mix and 0.25 μL TaqMan SNP Genotyping Assay Mix. After an initial denaturation at 95°C for 10 minutes, amplification was performed using 40 cycles of denaturation (92°C, 15 seconds), annealing (60°C, 1 minute), and extension (60°C, 1 minute). The PCR was performed using the Real-Time PCR StepOnePlus (Invitrogen).
Statistical analysis
Of the 1222 patients of the RICOVER-60 trial, DNA samples were available from 512. The allelic frequencies between lymphoma samples and controls were compared with Fisher exact test. Response was defined as the proportion of patients with complete remission or unconfirmed complete remission after study treatment for all patients evaluable for response. Response was assessed by use of Fisher exact test and Armitage trend test.30 Event-free survival (EFS) was defined as the time from the beginning of therapy to disease progression, relapse, death, or initiation of additional (off-protocol) or salvage therapy. Progression-free survival (PFS) was defined as time to disease progression, relapse, or death. Overall survival (OS) was defined as time to death for any reason. EFS, PFS, and OS were estimated with the Kaplan-Meier method and were compared using the log-rank test. Kaplan-Meier estimates at 3 years (with 95% confidence interval [CI]) were calculated for the probability of not having an event in the endpoints of EFS, PFS, and OS. Multivariate analyses were done using Cox proportional-hazard models to estimate hazard ratios for having an event. Differences between groups were regarded as significant for P values < .05 (2-sided). Statistical analyses were performed with R Version 2.931
Results
The results of the RICOVER-60 trial have been published previously. In a 2 × 2 randomized trial design, patients were randomized to 6 cycles of CHOP-14, 8 cycles of CHOP-14 without rituximab, or the same regimens plus 8 applications of rituximab every 2 weeks. The 2 arms with rituximab had a significantly improved EFS and PFS compared with 6 times CHOP-14 (P < .001), whereas only 6 cycles with 8 applications of rituximab, but not 8 cycles of CHOP-14 with 8 applications of rituximab, had a significantly improved OS compared with CHOP-14 (P = .018 and P = .260, respectively). The adherence to protocol was excellent: median relative doses for the myelosuppressive drugs were 98% or more (range, 1-118) for the 6-cycle regimens and 95% or more (range, 7-111) for the 8-cycle regimens, and median duration of chemotherapy cycles was 14 days (range, 8-92 days) in all regimens and cycles.
Prevalence of FcγRIIa and FcγRIIIa genotypes
Of 1222 elderly patients (61-80 years of age) recruited to the RICOVER-60 trial, samples for single nucleotide polymorphism (SNP) analysis were available from 512: 263 treated with R-CHOP-14 and 249 treated with CHOP-14 only. Their characteristics are shown in Table 1 and supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Except for a somewhat longer follow-up, these patients were representative of the entire RICOVER-60 population (supplemental Table 3). Among the 512 patients, 64 (12.5%) were carriers of FcγRIIa 131 R/R, 235 (45.9%) of R/H, and 213 (41.6%) of H/H. With respect to FcγRIIIa 158 polymorphism, the distribution was: V/V, 145 (28.3%); V/F, 271 (52.9%); and F/F, 96 (18.8%). The observed genotype frequencies did not deviate from those expected under the Hardy-Weinberg equilibrium. The genotypes of FcγRIIa 131 and FcγRIIIa 158 were only weakly linked (R2 = 0.026). The characteristics of patients from whom DNA was available for this study were not different for patients treated with R-CHOP and CHOP, respectively (supplemental Tables 1 and 2). Similarly, the genotype frequencies were similar for both treatment arms (supplemental Table 4). In the groups treated with CHOP, there was an imbalance with respect to bulky disease for the FcγRIIa polymorphism: R/R had 27%, R/H 54%, and H/H 34% (P = .003). In addition, there was a slight imbalance for the FcγRIIIa polymorphisms concerning age (P = .048) and Eastern Cooperative Oncology Group more than 1 (P = .038, supplemental Table 1) but not in the groups with R-CHOP. There were no significant differences between the FcγRIIa and FcγRIIIa polymorphisms in the groups treated with rituximab (supplemental Table 2). Given the large number of statistical tests in supplemental Tables 1 and 2, the observed imbalances might as well have occurred by chance. Blood samples of 101 consecutive blood donors of white origin were genotyped for FcγRIIa 131 and FcγRIIIa 158 and served as normal controls. Genotype distribution of FcγRIIa 131 was 15 (14.9%) for R/R, 48 (47.5%) for R/H, and 38 (37.6%) for H/H. For the FcγRIIIa 158 polymorphisms, the distribution was: V/V, 30 (29.7%); V/F, 48 (47.5%); and F/F, 23 (22.8%). Genotype frequencies of control samples did not deviate from those expected under Hardy-Weinberg equilibrium. No significant differences between genotype frequencies of lymphoma patients and normal controls were observed for either FcγRIIa 131 or FcγRIIIa 158 (data not shown).
Clinical presentation
Carriers of FcγRIIIa 158 V/V presented with a slightly decreased incidence of B-symptoms (158 V/V, 26%; V/F, 35%; F/F, 42%; P = .037). Apart from this, there were no associations of FcγRII/RIII carrier states with patient characteristics. In particular, carrier state of the various SNPs was not correlated with the International Prognostic Index risk factors, Eastern Cooperative Oncology Group performance status, elevated pretreatment lactate dehydrogenase, stage, and > 1 extranodal involvement or the International Prognostic Index score (Table 1).
Toxicity
With respect to the occurrence of side effects, there was a trend (Fisher exact test, P = .007; Armitage trend test, P = .091) that chemotherapy-induced grade 3 or 4 anemia was less frequent in FcγRIIa R/R (5 of 63 [toxicity data were missing from 1 patient] or 7.9%; 95% CI, 2.6-17.6) compared with FcγRIIa R/H (56 of 220 or 25.5%; 95% CI, 19.8-31.7) and FcγRIIa H/H (47 of 205 or 22.9%; 95% CI, 17.4-29.3). The rate of grade 3 or 4 leukocytopenias and thrombocytopenias as well as the rate of grade 3 or 4 infections was not different between the FcγRIIa and FcγRIIIa SNPs (data not shown). The rate of therapy-associated deaths was 10.9% (4.5%-21.2%) for FcγRIIa 131 R/R, 5.5% (3.0%-9.3%) for FcγRIIa 131 R/H, and 5.2% (2.6%-9.1%) for H/H (P = .220). There were no differences in therapy-associated deaths between the FcγRIIIa SNPs.
Response to therapy and outcome
The observation time was the same for all FcγRIIa and FcγRIIIa variants. The rates of complete remissions were not different between carriers of the FcγRIIa variants. With respect to the FcγRIIIa variants, the rates of CR/CRu for patients treated with CHOP only were 73.9% for 158 V/V, 77.1% for 158 V/F, and 63.3% for 158 F/F (Fisher exact test, P = .17; Armitage trend test, P = .27). The CR rates in patients treated with R-CHOP were 86.8% (158 V/V), 81.4% (158 V/F), and 74.5% (158 F/F; Fisher exact test, P = .23; Armitage trend test, P = .08; Table 2).
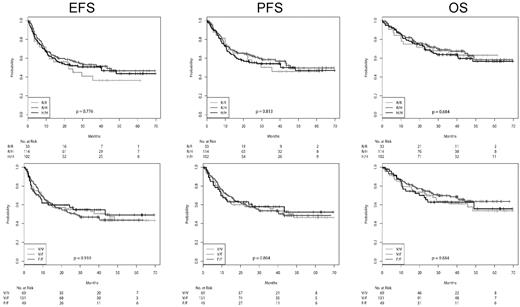
EFS, PFS, and OS, according to the FcγRIIa and FcγRIIIa genotypes for the patients receiving CHOP-14 and R-CHOP-14, respectively, are shown in Figures 1 and 2, and the number of events in the various subgroups is shown in supplemental Table 5. The adherence to protocol was excellent, with relative doses of the myelotoxic drugs cyclophosphamide and doxorubicin being 98% in the 6-cycle arms and 96% in the 8-cycles arms. There were no differences between FcγR polymorphisms. Similarly, there were also no differences with respect to the percentage of patients going off therapy or receiving nonprotocol therapy (data not shown). For patients receiving CHOP-14 (Figure 1), the survival curves of all SNPs were virtually superimposable. The survival curves according to the SNPs after R-CHOP-14 are shown in Figure 2. The survival curves were not different with respect to FcγRIIa variants, whether treated with R-CHOP or CHOP, respectively. In contrast, although there was also no difference of the survival curves with respect to FcγRIIIa variants for patients treated with CHOP, in patients treated with R-CHOP, the EFS and PFS, but not the OS, curves for FcγRIIIa 158 F/F showed a trend to be lower than those of 158 V/F and 158 V/V. The 3-year EFS rates for FcγRIIIa 158 F/F were 64.5% (50.5%-78.5%), compared with 70.2% (62.5%-78.0%) for FcγRIIIa 158 V/F and 76.9% (67.3%-86.6%) for FcγRIIIa 158 V/V (log-rank test, P = .224 for F/F vs V/V; P = .285 for F/F vs V/F + V/V). For 3-year PFS, the rates were 68.3% (54.6%-82.1%) for FcγRIIIa 158 F/F, compared with 76.1% (68.7%-83.5%) for FcγRIIIa 158 V/F and 80.5% (71.2%-89.7%) for FcγRIIIa 158 V/V (log-rank test, P = .233; for F/F vs V/V; P = .185 for F/F vs V/F + V/V).
EFS, PFS, and OS of patients receiving CHOP-14 according to FcγR polymorphisms. From left to right: EFS, PFS, and OS. Top row: FcγRIIa SNPs. Bottom row: FcγRIIIa SNPs. Survival curves were compared using the log-rank trend test.
EFS, PFS, and OS of patients receiving CHOP-14 according to FcγR polymorphisms. From left to right: EFS, PFS, and OS. Top row: FcγRIIa SNPs. Bottom row: FcγRIIIa SNPs. Survival curves were compared using the log-rank trend test.
EFS, PFS, and OS of patients receiving R-CHOP-14 according to FcγR polymorphisms. From left to right: EFS, PFS, and OS. Top row: FcγRIIa SNPs. Bottom row: FcγRIIIa SNPs. There is a clear trend for a worse EFS and PFS for patients with FcγRIIIa F/F.
EFS, PFS, and OS of patients receiving R-CHOP-14 according to FcγR polymorphisms. From left to right: EFS, PFS, and OS. Top row: FcγRIIa SNPs. Bottom row: FcγRIIIa SNPs. There is a clear trend for a worse EFS and PFS for patients with FcγRIIIa F/F.
Multivariate analysis
Based on the results of the univariate analysis, we analyzed the impact of FcγRIIIa 158 in the R-CHOP cohort using a multivariate Cox model with adjustment for International Prognostic Index factors. Comparing F/F versus V/F plus V/V, the relative risk for PFS was 1.80 (P = .052) for EFS 1.55 (P = .120) and 1.35 (P = .360) for OS. The results of the multivariate analysis within the R-CHOP cohort are shown in Table 3. To further investigate the interaction between rituximab and the FcγRIIIa 158 SNP, we used the complete dataset to fit a Cox model adjusting for rituximab, FcγRIIIa 158, and the interaction between both. The observed relative risks for the interaction were 1.57 (P = .24) for PFS, 1.47 (P = .28) for EFS, and 1.01 for OS (Table 4). Although not significant, these analyses indicate a trend toward an unfavorable outcome for carriers of FcγRIIIa 158 F/F under R-CHOP therapy. Given the observed effect sizes for the univariate analysis of the FcγRIIIa 158 SNP within the R-CHOP cohort, a post-hoc power analysis results in a power of 28% for PFS and 21% for EFS for the sample size of our R-CHOP cohort (n = 263). For a power of 80%, a sample size of n = 1100 (PFS) or n = 1600 (EFS) would be needed.
Discussion
FcγR polymorphisms were not associated with clinical features presented by patients with DLBCL, except for B-symptoms, which were less often present in patients carrying FcγRIIIa 158 V/V (P = .037). This observation, however, should be interpreted with caution because multiple clinical parameters were tested for association with FcγR polymorphisms, and thus, this association might have been observed by chance.
The focus of our study was to search for interactions between FcγR polymorphisms and outcome of patients treated with R-CHOP. It has been suggested that the level of CD20 expression on lymphoma cells,32 the presence of high tumor burden at the time of treatment,33 or low rituximab serum concentrations32,34 may explain the lack of efficacy of rituximab in some patients. Nevertheless, the actual causes of treatment failure are largely unknown. Cartron et al20 were the first to show that polymorphisms in the FcγRIIIa gene were also associated with the response to therapy in patients with follicular lymphoma with rituximab monotherapy. This was confirmed by Weng and Levy21 and was also observed by Ghielmini et al35 in patients with Waldenström macroglobulinemia.22 There are fewer reports showing a role of FcγRIIa polymorphisms.21 To the best of our knowledge, there are only 2 publications on the role of FcγR polymorphisms in DLBCL: in a study of Korean patients, FcγIIIa, but not FcγIIa, polymorphisms were associated with outcome after R-CHOP, whereas a small European study did not observe any differences with respect to FcγR alleles.28 Both studies suffer from relatively small numbers of patients included. Moreover, neither study restricted their analysis to patients treated uniformly within a prospective trial. This study is the first that is restricted to a single large prospective study, the RICOVER-60 trial.4 Although no influence of FcγRIIa on outcome of patients treated with CHOP-14 or R-CHOP-14 was observed, FcγIIIa alleles had an interaction with outcome after R-CHOP, but not CHOP, with FcγIIIa F/F being associated with a trend toward an inferior CR rate, EFS, and PFS. Despite the fact that this is by far the largest study on the role of FcγR polymorphisms in DLBCL, the differences between the different FcγRIIIa alleles did not reach significance. An extrapolation modeling showed that this would also not have been the case if blood samples of all 1222 DLBCL patients treated in the RICOVER-60 trial had been available: for a power of 80%, a sample size of 1100 patients for PFS and 1600 patients for EFS would have been needed. Nevertheless, the interaction between FcγIIIa and R-CHOP was relevant for PFS and EFS. The observed relative risks for the interaction were 1.57 (P = .24) for PFS and 1.47 (P = .28) for EFS (Table 4). Although not significant, these analyses indicate a trend toward an unfavorable outcome for carriers of FcγRIIIa 158 F/F under R-CHOP therapy. Although a similar trend was reported in R-CHOP-treated patients in the Korean study, the differences in outcome in our study with respect to FcγIIIa polymorphisms did not reach significance, despite the much higher number of patients included in this study. This might be because the Korean patients were treated with a thrice-weekly regimen and our patients with a twice-weekly application of rituximab. The higher rituximab serum levels achieved with the biweekly application (C. Müller, N. Murawski, G. Held, M. Wiesen, M. Wenger, C. Nickenig, N. Peter, E. Lengfelder, B. Metzner, M.P., and M. Reiser, Differences in rituximab clearance and serum elimination half life between elderly male and female patients with DLBCL, manuscript under review) might quench possible differences between Fc receptors with different binding affinities to rituximab; however, the interaction of the different pharmacokinetics of the twice-weekly and thrice-weekly application of rituximab and FcγR polymorphisms is unknown. Therefore, the differences between our study in white patients and the Korean study might be the result of other reasons (eg, other yet unidentified genetic differences). When one looks at studies that investigated FcγR polymorphisms in patients treated with monoclonal antibodies, it becomes evident that interactions between FcγIIIa polymorphisms and outcome have been observed repeatedly, even if antibody therapy was combined with chemotherapy. For FcγRIIa polymorphisms, this was not reported when the respective antibody was combined with cytotoxic agents.
In conclusion, we found a relevant interaction for FcγIIIa receptor polymorphisms with R-CHOP, but not with CHOP, treatment in this largest series of DLBCL patients treated uniformly within the randomized RICOVER-60 trial. Whether this relevant interaction translates into significant differences in outcome of patients with different FcγIIIa polymorphisms depends (in addition to the number of patients studied) on many factors, only some of which have been identified. The interaction between FcγIIIA receptor polymorphisms and rituximab, even when combined with CHOP chemotherapy, provides a more or less opened window of opportunity for therapeutic monoclonal antibodies engineered to overcome the differences in binding of their Fc parts to FcγRIIIa coded by distinct polymorphisms.36-38
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Deutsche Krebshilfe (15336).
National Institutes of Health
Authorship
Contribution: M.P. and K.-D.P. designed the study; M.A., J.B., and E.R. performed and evaluated the experiments; M.K. performed the statistical analysis; and M.A., M.K., and M.P. wrote the manuscript.
Conflict-of-interest disclosure: M.P. has received honoraria and research support from Roche and Amgen and is on the advisory boards of Roche, Sanofi, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Michael Pfreundschuh, Saarland University Medical School, D-66421 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.