In this issue of Blood, Scholz et al have demonstrated that the expression of angiopoietin-2 (Ang2) in the endothelial cells is sufficient to recruit myeloid cells and induce inflammation even in the absence of preceding proinflammatory stimuli.1 This finding shows that the endothelial cells are capable of leading and orchestrating the whole inflammatory process via Ang2.
Along with the VEGF-VEGFR system, the angiopoietin-Tie system is a well-known endothelial cell–specific ligand-receptor axis that regulates vasculogenesis, angiogenesis, and vessel maturation. The angiopoietin family consists of 4 different secretory proteins, of which Ang1 and Ang2 have gained the most attention and have been extensively studied for their roles in angiogenesis. Ang1 and Ang2 are agonistic and antagonistic Tie2 ligands, respectively (reviewed in Augustin et al2 ), and Ang2 in particular has recently been highlighted as an attractive therapeutic target for anti-angiogenic therapy, particularly in the field of oncology. For instance, one study using a specific Ang2-blocking antibody reported encouraging results with tumor growth inhibition and metastasis reduction in experimental animals, the result of impaired angiogenesis and disabled rebounds of proangiogenic myeloid cells.3 Nevertheless, much remains unknown about Ang2.
Accumulating evidence indicates that angiogenic molecules often serve as critical links in various disease conditions such as inflammation and cancer (reviewed in Augustin et al2 ). Intuitively, this is a reasonable notion because the onset and progression of such diseases would inevitably require new blood vessel formation, while the disease state itself continues to exercise significant influence on the permeability of the nearby vasculature. Moreover, some studies showed that the angiopoietin- Tie system is profoundly involved in the pathogenesis of inflammation4,5 : it has been reported that Ang2-deficient mice display defects in generation of rapid inflammatory responses, and the state of Ang2 deficiency influences leukocyte firm adhesion and transmigration.2,4 However, it still remained uncertain until now whether the angiogenic signals are direct regulators of the inflammatory process, or simple mediators that merely propagate and/or amplify other preceding proinflammatory stimuli.
Here, Scholz et al demonstrate that the up-regulation of Ang2 itself can serve as a primary cue for recruiting myeloid cells to instigate inflammation, which they claim to be achieved in a β2 integrin-dependent manner.1 The authors analyzed biopsy samples from patients of inflammatory disorders and found that the vessels near the regions infiltrated by monocytes/macrophages frequently displayed robust Ang2 expression, which led them to speculate that the up-regulated Ang2 expression could be a critical step in the initiation of inflammatory process. To verify their hypothesis, Scholz et al used double transgenic mice (Tie1 tTA driver and Ang-2TetOS responder) that were designed to overexpress Ang2 specifically in the endothelial cells in a drug-inducible manner. These mice developed signs of systemic inflammation, with multiple organs displaying infiltration of myeloid cells. In addition, the authors observed that the Ang2-overexpressing mice were relatively vulnerable to inflammatory stimuli compared with wild-type controls, as the amplitude of inflammation was more severe when the mice were challenged with Dinitro-fluorobenzene and thioglycollate.
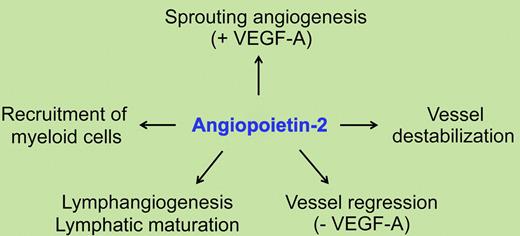
Collectively, this study provides compelling evidence that the up-regulation of Ang2 has an important role in the generation of inflammatory responses, and that the over-expression of Ang2 alone is sufficient to induce inflammation even in the absence of other preceding inflammatory stimuli. This adds another line to the list of Tie2- and integrin-mediated Ang2 functions, which can now be summarized as sprouting angiogenesis, vessel destabilization, and regression (reviewed in Augustin et al2 ), and instigation of inflammation1 (see figure).
It is interesting that the counteracting relationship of Ang1 and Ang2, as seen in angiogenesis, is also valid in the regulation of inflammation: Ang1 seems to have an anti-inflammatory role,6,7 while Ang2 works opposite by exerting a proinflammatory effect.1,4 This bi-directional system is expected to establish a balanced regulatory circuit that allows delicate and efficient control over inflammation.
The concept of Ang2 as an agent of inflammatory process has important clinical implications, considering the intimate relationship of inflammation with various diseases such as atherosclerosis (reviewed in Ross8 ). Because endothelial cells are the predominant site of Ang2 production, the role of Ang2 as an independent trigger of inflammation implies that endothelial cells do not merely serve as a linker that transmits noxious stimuli for the propagation of inflammation, but can act as the ringleader of the whole inflammatory process under appropriate circumstances. Therefore, Ang2 should be recognized and vigorously assessed as a promising therapeutic target for elimination of the initial proinflammatory focus.
However, questions such as whether reciprocal and/or compensatory mechanisms exist between the angiopoietin family and the VEGF signaling pathways during the Ang2-induced inflammation activation process, remain to be answered. Can the lack ofAng2 up-regulation be further verified in earlier periods of lymphoid-dominant inflammation to supplement the findings of this study? Scholz et al describe myeloid cell infiltration as a major outcome of Ang2 expression and a lack of Ang2 up-regulation in biopsy specimens obtained from patients of lymphoid-dominant diseases; yet myeloid cell infiltration, although transient, often does occur before lymphoid cell recruitment during the initial stages of lymphoid dominant inflammation, which unfortunately lie within a time period in which biopsy is rarely considered. It would be interesting if the Ang2 levels could be examined during earlier but more dynamic periods when the myeloid cells are temporally recruited into the inflamed tissue.
In summary, Scholz et al identify Ang2 as a crucial molecule that is capable of independently initiating and leading the inflammation process to cause myeloid cell recruitment. This shows that the direct influence of Ang2 could be much more extensive than previously believed. We hope that subsequent studies will further elucidate the role of Ang2 and promote the clinical application of Ang2-modulating therapies.
Conflict of interest disclosure: The authors declare no competing financial interests. ■