Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is a genetic disorder characterized by early-onset, chronic, nonmalignant lymphoproliferation, autoimmune manifestations, and susceptibility to lymphoma. The majority of ALPS patients carry heterozygous germline (ALPS-FAS) or somatic mutations (ALPS-sFAS) of the TNFRSF6 gene coding for FAS. Although the clinical features of ALPS have been described previously, long-term follow-up data on morbidity and mortality are scarce. We performed a retrospective analysis of clinical and genetic features of 90 ALPS-FAS and ALPS-sFAS patients monitored over a median period of 20.5 years. Heterozygous germline mutations of TNFRSF6 were identified in 83% of probands. Somatic TNFRSF6 mutations were found in 17% of index cases (all located within the intracellular domain of FAS). Sixty percent of the ALPS-FAS patients with mutations in the extracellular domain had a somatic mutation affecting the second allele of TNFRSF6; age at onset was later in these patients. No other genotype-phenotype correlations could be found. Long-term analysis confirmed a trend toward spontaneous remission of lymphoproliferation in adulthood but mixed outcomes for autoimmune manifestations. We observed significant and potentially life-threatening disease and treatment-related morbidity, including a high risk of sepsis after splenectomy that calls for careful long-term monitoring of ALPS patients. We also noted a significantly greater occurrence of disease-related symptoms in male than in female patients.
Introduction
Autoimmune lymphoproliferative syndrome (ALPS, also known as Canale-Smith syndrome)1 is a genetic disorder characterized by early-onset, chronic, nonmalignant lymphoproliferation, autoimmune manifestations, and susceptibility to lymphoma.2 In ALPS, lymphocyte homeostasis is disrupted by defects in the apoptosis mediated by Fas, a cell-surface receptor (also referred to as Apo-1 and CD95) from the TNF receptor superfamily.3,4 High counts of circulating TCRαβ+, CD4−CD8− double-negative (DN) T lymphocytes and elevated plasma levels of Fas ligand (FasL) and IL-10 are diagnostic hallmarks of the disease.5 The majority of ALPS patients carry heterozygous germline mutations in the TNFRSF6 gene coding for Fas6-8 (ALPS-FAS). Interestingly, somatic TNFRSF6 mutations represent the second most common genetic etiology of ALPS (ALPS-sFAS).9,10 In addition, germline mutations in the genes coding for Fas L, caspase 10, and caspase 8 and somatic mutations in NRAS and KRAS have been identified in a small number of patients with ALPS and related disorders.11-16 The classification and diagnostic criteria for ALPS were revised recently.17
The majority of ALPS-FAS–related mutations are located in the Fas intracellular domain (ICD) and within its death domain (DD) in particular. The reported clinical penetrance of ICD mutations is high (> 80%) because the mutant proteins exert dominant-negative effects on wild-type CD95 protein.18-20 In contrast, mutations located in the Fas extracellular domain (ECD) usually display lower clinical penetrance (20%-30%) because of haploinsufficiency. These observations suggest that a second event is necessary for disease expression in such cases. In fact, we recently reported on 7 patients with both a germline ECD mutation and a somatic event affecting the second TNFRSF6 allele (a somatic mutation or duplication of the mutant allele with loss of the wild-type allele).19 The clinical phenotype of affected relatives is also reportedly milder in families with ECD mutations.18 Although the clinical features of ALPS have been described previously,18,20-23 data on long-term morbidity and mortality are scarce. In the present study, we report on the clinical and genetic features of 79 ALPS-FAS and 11 ALPS-sFAS patients monitored over a median follow-up period of 20.5 years (range: 1.5-77 years).
Methods
Inclusion criteria
All index cases were referred to the Primary Immunodeficiency Study Center at Necker Children's Hospital (Paris, France) due to suspected ALPS (defined as chronic, nonmalignant lymphadenopathy and/or splenomegaly with an elevated DN T-cell count (CD3+ TCRαβ+ CD4−CD8− ≥ 2.5%) in the peripheral blood.17 Patients with defective in vitro Fas-induced lymphocyte apoptosis and/or elevated plasma FasL were screened for germline TNFRSF6 mutations using genomic DNA. If no mutations were found, genomic DNA extracted from sorted DN T cells was sequenced as a screen for mosaic mutations. Only patients with confirmed, heterozygous, germline mutations (ALPS-FAS) and somatic TNFRSF6 mutations (ALPS-sFAS) were included in this study. All participants or their parents/guardians gave signed, informed consent to participation in accordance with the Declaration of Helsinki. Patients were subsequently cared for in 18 medical centers across France and Belgium. Clinical data were collected from medical charts (reviewed by 2 physicians: B.N. and B.F.) and in physician interviews.
Relatives of index cases underwent genetic and immunologic screening. Laboratory tests (eg, detection of DN T cells and plasma FasL and in vitro lymphocyte T-cell apoptosis assays) were performed whenever possible. Phenotypic features (splenomegaly, adenopathy, and overt autoimmunity) were described by reviewing medical records (when available) and/or performing physician interviews. On the basis of this information, relatives were classified as asymptomatic carriers or symptomatic relatives. Three first-degree relatives with ALPS-related symptoms (ie, chronic splenomegaly and/or lymphadenopathy with autoimmunity and/or lymphoma on one occasion) were included in the study despite the absence of data on TNFRSF6 mutations. Another 2 relatives were excluded from the study due to a lack of medical information. The study end date for inclusion and data collection was September 2010.
Diagnostic procedures
The percentage of DN T cells, plasma IL-10 and FasL levels, and lymphocyte T-cell apoptosis were assessed as described previously.19 For the mutation analysis, DNA was isolated by proteinase K digestion and phenol-chloroform extraction. Genomic DNA segments were amplified as described previously.22 In families with incomplete clinical penetrance of germline TNFRSF6 mutations or in patients with de novo germinal mutations in the Fas ECD, additional somatic TNFRSF6 mutations were screened for after DN T-cell sorting, as described previously.19
Statistics
All statistical analyses were performed using Prism 4 software (GraphPad). Groups were compared in a Mann-Whitney test.
Results
Identification of TNFRSF6 mutations in index cases
A total of 63 index cases were analyzed. Fifty-two probands (82%) carried a heterozygous germline TNFRSF6 mutation. There were 12 probands with ECD mutations (23%), 1 with a transmembrane domain (TMD) mutation, and 39 with ICD mutations (75%), as shown in Figure 1A and B. Ten of these ECD mutations had not been reported elsewhere and are highlighted in Figure 1A. Six of the ICD mutations were found in at least 2 unrelated index cases. In total, there were 26 different ICD mutations, 14 of which had not been reported elsewhere (Figure 1B). In addition, 11 patients had mosaic ALPS (18%) and displayed somatic TNFRSF6 mutations (all in the ICD) in DN T cells (Figure 1C). Somatic changes in the second TNFRSF6 allele were screened for in 10 of the 13 ALPS index cases carrying ECD/TMD mutations and were identified in 6 of these (60%). As reported previously,19 a somatic mutation affecting the second TNFRSF6 allele was found in 2 patients. Duplication of the mutant allele with loss of the wild-type allele was identified in 4 cases (2 of which had been reported previously19 ). Six of the 39 index cases with germline ICD mutations and low clinical penetrance were screened for somatic changes of the second TNFRSF6 allele. Loss of the wild-type allele was identified in 1 of these cases.
Genotypic characteristics of ALPS-FAS and ALPS-sFAS patients. The structure of the TNFRSF6 gene showing ALPS-FAS–associated mutations in exons 1-6 (A) encoding the extracellular domain (ECD) and transmembrane domain (TMD) and exons 7-9 (B) encoding the intracellular domain (ICD). Mutations are numbered with respect to the immature protein. The symbols denote missense (●) and frameshift mutations ( ); deletions and nonsense mutations are also indicated. Mutations denoted with an asterisk are novel. The numbers of unrelated index cases carrying the same mutation are given in brackets. Mutations associated with ALPS-sFAS are shown in panel C.
); deletions and nonsense mutations are also indicated. Mutations denoted with an asterisk are novel. The numbers of unrelated index cases carrying the same mutation are given in brackets. Mutations associated with ALPS-sFAS are shown in panel C.
Genotypic characteristics of ALPS-FAS and ALPS-sFAS patients. The structure of the TNFRSF6 gene showing ALPS-FAS–associated mutations in exons 1-6 (A) encoding the extracellular domain (ECD) and transmembrane domain (TMD) and exons 7-9 (B) encoding the intracellular domain (ICD). Mutations are numbered with respect to the immature protein. The symbols denote missense (●) and frameshift mutations ( ); deletions and nonsense mutations are also indicated. Mutations denoted with an asterisk are novel. The numbers of unrelated index cases carrying the same mutation are given in brackets. Mutations associated with ALPS-sFAS are shown in panel C.
); deletions and nonsense mutations are also indicated. Mutations denoted with an asterisk are novel. The numbers of unrelated index cases carrying the same mutation are given in brackets. Mutations associated with ALPS-sFAS are shown in panel C.
Family analysis
Genetic analysis of family members was performed for 46 of the 52 patients carrying germline TNFRSF6 mutations. No clinical or immunologic features of ALPS were detected in the parents of the 9 patients with de novo germline mutations. Inherited mutations were found in 37 families, with maternal and paternal inheritance in 19 and 18 cases, respectively. The mutation was also detected in 5 grandparents, 17 siblings, 3 children, and 9 other relatives. Of the 71 individuals with germline TNFRSF6 mutations, 24 had a positive medical history and biologic markers of ALPS, whereas 40 adults and 7 children were asymptomatic. Two relatives (1 grandfather and 1 sibling) also had a typical medical history of ALPS but were not available for genetic testing. Six families were not investigated. In one of the latter families, medical history was suggestive of ALPS; the proband's mother had presented with Hodgkin lymphoma (HL) in childhood and benign lymphoproliferation in adulthood. In total, 27 symptomatic relatives were identified and were included in the clinical survey.
Seven symptomatic relatives carried ECD/TMD mutations. Somatic changes in the second TNFRSF6 allele were screened for in 3 of these subjects and confirmed in 2. Both cases corresponded to duplication of the mutant allele with loss of the wild-type allele.
Clinical features
The demographic and clinical characteristics of the patient population are summarized in Table 1. At last follow-up, the median age of all patients (ie, index cases and symptomatic relatives) was 22.5 years (range: 1.9-77 years). The male/female gender ratio was 2.2. Index cases (n = 63) had a median age of 17.6 years (1.9-48 years) with a gender ratio of 2.1. Symptomatic relatives (n = 27) had a median age of 37.5 years (1.2-77 years) and a gender ratio of 2. Asymptomatic family members had a median age of 43.7 years (5-73 years) and a gender ratio of 0.7. Higher penetrance in males was suggested by the fact that 75% of the genetically affected males developed ALPS compared with 50.8% of females (P < .01).
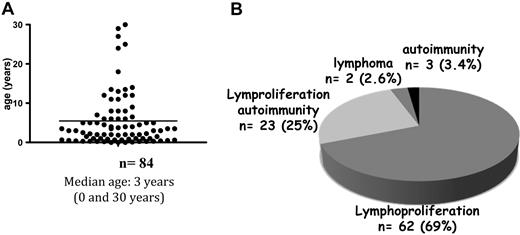
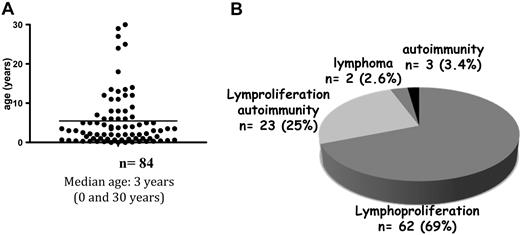
The median age at symptom onset was 3 years (range: 0-35; Figure 2A). However, 7 of the 90 patients (7%) had late-onset disease (ie, between the ages of 18 and 35 years.) Patients with late-onset disease displayed milder lymphoproliferation but showed active autoimmune manifestations. The first disease manifestations are depicted in Figure 2B.
Age at onset and the nature of the first manifestations of ALPS. (A) Age distribution at disease onset for 84 patients. (B) Distribution of the first symptoms of ALPS.
Age at onset and the nature of the first manifestations of ALPS. (A) Age distribution at disease onset for 84 patients. (B) Distribution of the first symptoms of ALPS.
Lymphoproliferation
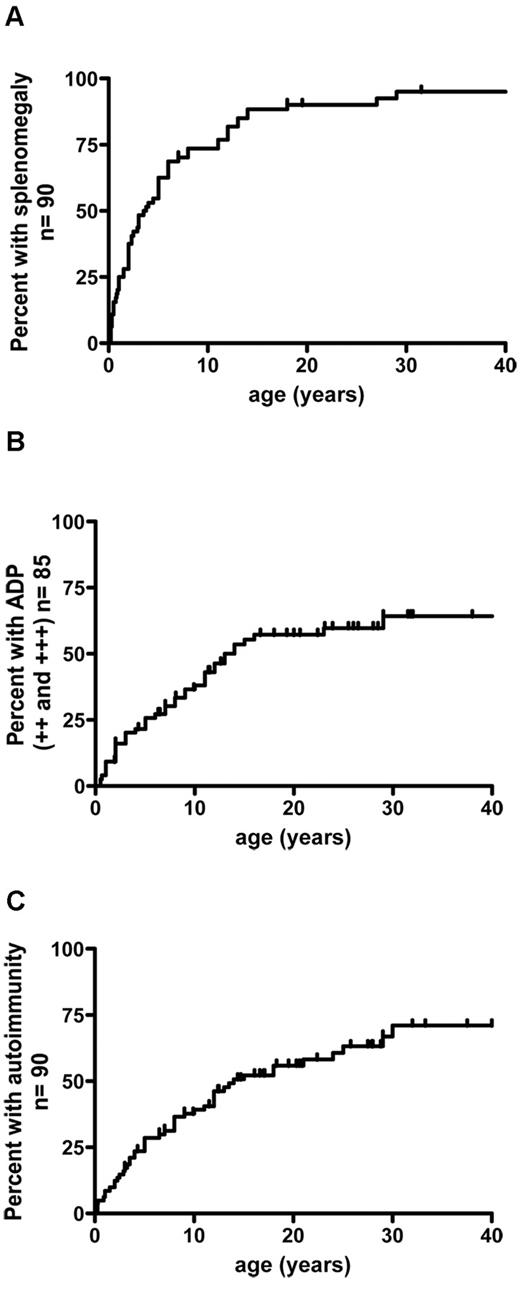
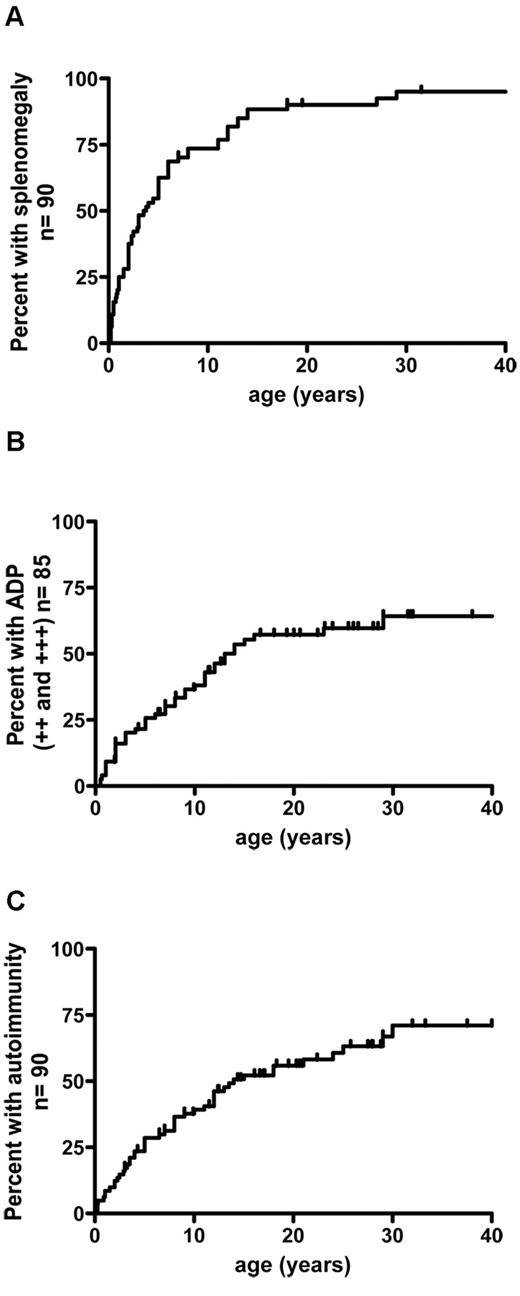
Chronic lymphoproliferation was present in 89 of the 90 subjects. Splenomegaly was the predominant feature (n = 85, 94%) and generally occurred early in the course of the disease (Figure 3A). Hypersplenism was noted in 70% of the patients (58 of 80). Splenic fracture occurred in 1 individual. One patient presented hydrops fetalis at birth as a consequence of massive prenatal lymphoproliferation and cytopenia. Chronic lymphadenopathy was also frequent (in 72 of 85 patients) but developed later than did splenomegaly (Figure 3B). Two patients presented with dysphonia related to histologically confirmed laryngeal lymphoproliferation.
Cumulative frequency of patients having developed lymphoproliferation and autoimmunity as a function of age. (A) Splenomegaly precedes the development of lymphadenopathy and autoimmune manifestations. (B) Lymphodemopathy develops in 72% of patients. (C) Autoimmunity occured in 75% of patients. ++ indicates lymphadenopathy > 2 cm and < 5 cm; +++, lymphadenopathy > 5 cm; and ADP, lymphadenopathy.
Cumulative frequency of patients having developed lymphoproliferation and autoimmunity as a function of age. (A) Splenomegaly precedes the development of lymphadenopathy and autoimmune manifestations. (B) Lymphodemopathy develops in 72% of patients. (C) Autoimmunity occured in 75% of patients. ++ indicates lymphadenopathy > 2 cm and < 5 cm; +++, lymphadenopathy > 5 cm; and ADP, lymphadenopathy.
Autoimmunity
Autoimmune manifestations occurred in 55 of the 90 patients (61%) at a median age of 6.25 years (range: 0-30 years). The risk of developing autoimmunity before the age of 30 was calculated to be 72% (Figure 3C). A detailed analysis of the autoimmune manifestations is provided in Table 2. Autoimmune cytopenia was the main manifestation and occurred in 47 of the 90 patients (52%) at a median age of 5 years (range: 0.25-30). Autoimmune hemolytic anaemia was the most frequent event (n = 30). Twenty-three patients had autoimmune thrombocytopenia (platelet count < 30 000/μL). Profound autoimmune neutropenia was diagnosed in 7 cases (< 200/μL). Sixteen other (later-onset) autoimmune manifestations occurred in 12 patients (14.4%) at a median age of 12.5 years (range: 0.25-24 years). Five of the latter events were preceded by or occurred concomitantly with autoimmune cytopenia. Two patients developed glomerulonephritis, with spontaneous recovery from transient acute kidney failure in one case and nephrotic syndrome requiring therapy in the other. Kidney biopsies from both patients revealed mesangiopathic glomerulonephritis with crescent formation and minor IgA deposits. Three patients presented with acute seronegative hepatitis, which resolved either spontaneously (n = 2) or after a short course of steroids (n = 1). Aplastic anaemia occurred in 2 patients, with nodular lymphoid infiltration (mostly by DN T cells). Chronic pancreatitis occurred in 1 patient. Another patient presented with severe osteopenia at the age of 6 years (osteodensitometry of the femur and spine: −7 SD), which led to pain, multiple bone fractures, and growth failure. This patient has never been treated with steroids. Osteopenia dramatically improved after immunosuppressive therapy that included cyclophosphamide, vincristine, steroids, and alemtuzumab. Hematopoietic stem cell transplantation (HSCT) was attempted but the graft was rejected. Significant improvement of osteopenia after immunosuppression suggested an autoimmune origin. Concomitant pulmonary and skin vasculitis occurred in 1 patient. Partial, transient alopecia was noted once. One patient presented recurrent angioedema and urticaria. Lastly, recurrent episodes of rash (reported as polymorphic maculopapular rash or giant urticaria) were reported in 20% of the patients.
Laboratory results
Data on IgG, IgA, and IgM serum levels were available for 73 patients. Almost all patients presented hyper-IgG (71 of 73) and hyper-IgA serum levels, although the phenotype varied markedly from one patient to another and over time for a given individual (data not shown). Two patients presented with a progressive hypo-IgG at the age of 15 years, both of whom required Ig substitution; neither of these 2 patients received previous immunosuppressive drugs despite active lymphoproliferation from infancy. IgM values were within the normal range in 35 of the 73 patients and below the normal range in 38. Hypo-IgM serum level was present at diagnosis in 1/2 of the cases and appeared over time in the other cases.
Disease management
Sixty-four of 86 patients (74%) required medical and/or surgical treatment at some point during the follow-up period. Thirty patients (33%) underwent complete splenectomy due to massive splenomegaly (n = 12) and/or refractory cytopenia/autoimmunity (n = 18). Nineteen of the 63 index cases (30%) were splenectomized at a median age of 6.5 years (range: 0.5-17 years) and 11 of the 27 symptomatic relatives (41%) at a median age of 15 years (range: 2-43 years). Splenectomy was initially efficient at treating cytopenia, but relapse of autoimmunity occurred in 1/2 of the patients. Fifty-two patients (60%) were treated with immunosuppressive drugs. Immunosuppression was primarily initiated due to autoimmunity in 80% and for lymphoproliferation (abdominal pain related to voluminous splenomegaly and/or massive lymphadenopathies) in the remaining 20%. Median age at initiation of medical treatment was 7.5 years (mean: 9.1, range: 0.1-29) for a median duration of 3 years (mean: 5.5, range: 0.5-23). The various immunosuppressive treatments administered were based mainly on steroids (n = 42), 6-mercaptopurine (6MP; n = 25), azathioprine (n = 19), mycophenolate mofetil (MMF; n = 5), rapamycin (n = 3), and anti-CD20 mAbs (n = 9). Cyclophosphamide pulse (n = 3) and plasmapheresis (n = 2) were rarely attempted in severe and refractory autoimmunity: autoimmune hemolytic anemia (AIHA), immune thrombocytopenic purpura (ITP), severe osteopenia, and hyperviscous syndrome related to hypergammaglobulinemia. HSCT was attempted in one patient for severe osteopenia (see “Autoimmunity”) but the graft was rejected. Efficacy of immunosuppressive drugs cannot be analyzed in deep in such a retrospective analysis in which several therapeutic modalities were used. It can nevertheless be restated that steroids were the front-line therapy for autoimmune manifestations. Second-line therapy was mainly 6MP, azathioprine, or MMF. Additional lines of therapy included anti-CD20 mAb, rapamycin, or combination of therapies. In the past, splenectomy was initially proposed as second- or third-line of treatment; however, in recent years, this procedure has been avoided due to the increased risk of invasive infection with encapsulated bacteria in this setting of patients (see next paragraph). Among 45 patients treated for autoimmunity, 7 received one line of therapy, 13 patients had 2, and 25 patients ≥ 3. Anti-CD20 mAbs were used in 9 patients with autoimmune cytopenia. As reported previously,24 efficacy in the treatment of AIHA was disappointing (no effect, n = 3; partial improvement, n = 3). Three patients with ITP achieved remission after rituximab, but 2 relapsed a few months later. Lymphoproliferation and hypersplenism were efficiently treated with 6MP, azathioprine, or, on a few occasions, sirolimus.
Malignancy
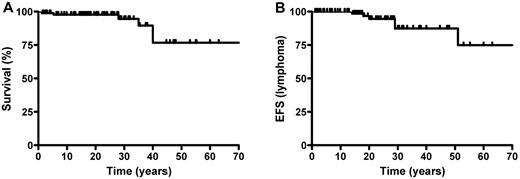
A total of 7 lymphomas occurred in 3 index cases and 3 symptomatic relatives. Median age at diagnosis was 24.5 years (range: 14-51). There were 3 cases of HL and 4 cases of B-cell nonHodgkin lymphoma (NHL). One case of B-cell NHL has been reported previously.25 One patient presented with concomitant large B-cell NHL in the duodenum and HL in the BM. One B-cell NHL case was EBV positive. All but 1 of the 6 patients had been treated for ALPS-related symptoms from early childhood on. One patient had presented with mild, undiagnosed lymphoproliferation before developing a lymphoid malignancy. All patients were treated with conventional chemotherapy, except for the case of EBV-related lymphoma, in whom only immunotherapy was administered (anti-CD20 mAbs and reduction of the immunosuppression). All patients with a follow-up of > 5 years (n = 4) showed long-term remission. The cumulative risk of developing lymphoid malignancy before the age of 30 years was calculated to be 15% (Figure 4B). Low-grade glioma occurred in 1 patient at the age of 19. It was successfully treated by radiotherapy, with a follow-up of 5 years.
Event-free survival and lymphoma-free survival. (A) Event-free survival and (B) lymphoma-free survival.
Event-free survival and lymphoma-free survival. (A) Event-free survival and (B) lymphoma-free survival.
Long-term disease progression
We analyzed the long-term data for a subgroup of informative patients who were symptomatic before the age of 15 and followed up beyond the age of 20 years. Twenty-six index cases and 16 symptomatic relatives met these criteria (n = 42). The data are shown in Table 3. The median duration of follow-up for these 42 patients was 28 years (mean: 32; range: 20.5-77 years). All 42 subjects presented with lymphoproliferation (100%), and 25 (59%) developed autoimmune phenomena during childhood. Twenty received drug treatment during childhood (48%). Lymphoproliferation improved in all subjects, with complete remission in 28 cases (66%) and significant improvement in the others (34%). Remission of autoimmunity was observed in 44% of cases. Autoimmune cytopenia remained active in 14 of 25 patients (56%). Although autoimmune manifestations were present in a milder form in 1/2 of them, all required continuation of immunosuppressive drugs in adulthood. Overall, these data confirmed a trend toward the progressive remission of lymphoproliferation in adulthood. However, autoimmune manifestations remained active and required long-lasting immunosuppressive drugs in adulthood.
Disease activity and treatment-related events
Nine of the 30 splenectomized patients (30%) developed 17 severe, invasive bacterial infections. Only 1 patient was receiving immunosuppressive treatment when infection occurred. Four patients died as a consequence of these infections, giving a mortality rate for invasive bacterial infection after splenectomy of 13.3%. The infections occurred 1.8-44 years after splenectomy (median: 10 years). The main pathogen was Streptococcus pneumoniae. Streptococcus agalactiae was identified in 1 adult male patient. No microorganisms were identified in 3 cases. Age at splenectomy appeared to be a significant risk factor for invasive bacterial infections: 4 of 6 patients splenectomized at ≤ 5 years of age developed 1-5 invasive bacterial infections, whereas 5 of 24 patients splenectomized at ≥ 5 years of age developed 1 episode of invasive bacterial infections (P < .05). There was also a trend for increased risk of invasive bacterial infection in patients with poor adherence to usual prophylactic recommendation (anti-bioprophylaxis during a minimum of 5 years after splenectomy in children, 2 years in adults, and updated vaccinations against S pneumoniae, H influenzae, and Neisseria meningitidis26 ). Three of 6 noncompliant patients versus 6 of 21 adherent patients developed invasive bacterial infections. Immunosuppressive treatment before or at the time of infection occurrence did not appear as a risk factor. Only 1 patient was receiving 6MP at onset of infection. Five of 16 patients who received immunosuppression before or after splenectomy developed an invasive bacterial infection versus 4 of 14 who did not received any immunosuppression. Considering splenectomized patients without any risk factors (> 5 years at splenectomy and good compliance to prophylactic recommendations), 3 of 18 (17%) patients developed infections. Subcutaneous abscesses were observed in 1 patient suffering from autoimmune neutropenia. Pneumocystis jiroveci pneumonia occurred in 2 patients on immunosuppressants (steroids, 6MP, azathioprine, and anti-CD20 mAb in one; steroids, 6MP, and anti-CD20 mAb in the other). Epidermodysplasia verruciformis (related to chronic human papillomavirus type 5 infection) occurred in 1 patient 4 years after an unsuccessful HSCT while she was being treated with 6MP. The patients did not suffer from any other recurrent or severe infectious complications.
Growth failure was observed in 21 index cases (33%). In 3 cases, growth failure occurred early (before the age of 5) and concomitantly with severe autoimmunity. This condition was treated with long-term steroid therapy. One patient had a growth arrest at the age of 6 years as a consequence of severe (and presumably autoimmune) osteopenia (“Auto immunity”). Among 17 patients with short stature and delayed puberty in the teenage years, 5 received long-term low-dose steroids beginning in early childhood (< 0.3 mg/kg/d) and ongoing in the teenage years. Five patients never received any steroids and 7 received short-course steroids or steroids > 3 months but only in early childhood (< 8 years old). None received pulse steroids. When considering index cases over the age of 20 years at last follow-up (n = 26), 11 of 26 (42%) had growth retardation during adolescence. All presented with catch-up growth in parallel to the development of puberty with adult height in the normal range.
Six patients (3 index cases and 3 symptomatic relatives) died during the study period at a median age of 25 years (range: 1.5-40). The main cause of death was postsplenectomy infection (n = 4). One patient with aplastic anaemia died at the age of 18 months. The third patient died of a stroke at the age of 35 years (Figure 4B).
Analysis of genotype/phenotype correlations and penetrance
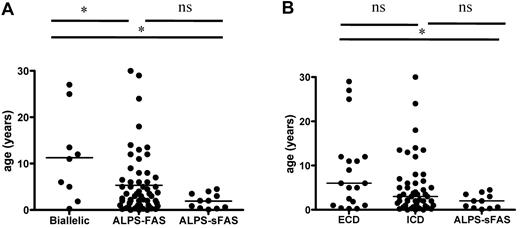
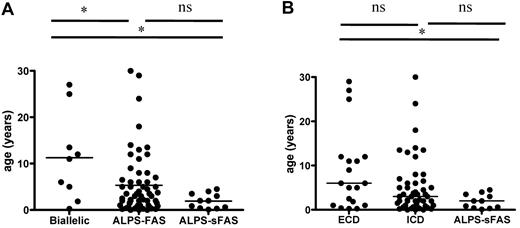
We analyzed the cohort's clinical parameters as a function of genotype (germline ECD and TMD mutations, n = 20; germline ICD, n = 59; and mosaic ICD, n = 11), as shown in Table 4. ALPS-FAS patients with associated somatic alterations of the second TNFRSF6 allele were also analyzed separately. Age at disease onset as a function of genotype is shown in Figure 5. Patients with combined germline and somatic TNFRSF6 mutations tended to be older at disease onset than other ALPS-FAS patients (P = .045) or ALPS-sFAS patients (P = .01). Similarly, patients with germline ECD mutations tended to be older than patients with ICD mutations and ALPS-sFAS at the time of occurrence of the first disease-related symptoms. The groups did not differ in terms of the presence of autoimmune manifestations, as shown in Table 4. Lymphoma occurred in patients from each of the genetic groups. Likewise, the groups did not differ in terms of clinical parameters (eg, lymphoproliferation and autoimmunity) or disease severity (eg, significant morbidity and need for treatment and remission). Interestingly, there was a male predominance among symptomatic individuals regardless of the genotype. A male/female gender ratio of 2.9 was observed for patients with autoimmune manifestations. In patients with autoimmune cytopenia, this male predominance was even stronger at 4.2 (M/F, 38/9). The gender ratio in patients presenting other autoimmune manifestations was 1.4 (M/F, 7/5). Penetrance among relatives and overall penetrance (for all mutation-positive index cases and relatives) are shown in Table 4. Missense mutations of exon 9 (encoding the DD) showed the highest penetrance. It is noteworthy that wide phenotype variability was observed for ALPS patients from the same family.
Age at onset of ALPS as a function of genotype. (A) Patients with germline TNFRSF6 mutation and a somatic event impairing the second TNFRSF6 allele (“biallelic” patients) had a later onset than germline-only ALPS-FAS or ALPS-sFAS patients. (B) ALPS-FAS patients with germline ECD mutations (including patients with TNFRSF6 biallelism) tended to be older at ALPS onset than patients with ICD mutation, although this difference was not statistically significant. ALPS-sFAS patients had the lowest age at ALPS onset. *P < .05; ns indicates nonsignificant.
Age at onset of ALPS as a function of genotype. (A) Patients with germline TNFRSF6 mutation and a somatic event impairing the second TNFRSF6 allele (“biallelic” patients) had a later onset than germline-only ALPS-FAS or ALPS-sFAS patients. (B) ALPS-FAS patients with germline ECD mutations (including patients with TNFRSF6 biallelism) tended to be older at ALPS onset than patients with ICD mutation, although this difference was not statistically significant. ALPS-sFAS patients had the lowest age at ALPS onset. *P < .05; ns indicates nonsignificant.
Discussion
We have reported the long-term phenotypic and genotypic characteristics of the largest cohort to date of heterozygous ALPS-FAS and ALPS-sFAS patients. Unsurprisingly, lymphoproliferation and autoimmunity were the hallmarks of the disease. Our long-term follow-up study confirmed a trend toward spontaneous remission of lymphoproliferation in adulthood. This trend was not clearly observed for autoimmune manifestations that persisted in a significant number of patients and required long-lasting immunosuppression at adulthood. We observed significant, life-threatening disease- and treatment-related morbidity. Strikingly, 17% of the index cases carried somatic TNFRSF6 mutations (all of which were located in the ICD). In accordance with previous reports, 25% of the heterozygous ALPS-FAS patients had mutations in the ECD and 75% had mutations in the ICD (with 29 of 40 in the DD).19 No genotype-phenotype correlations could be found other than later age at onset in patients with combined germline TNFRSF6 mutation and a somatic genetic event affecting the second TNFRSF6 allele. We also noted a significantly greater increase in disease-related symptoms in males than in females.
Lymphoproliferation was the most common sign of disease onset and generally occurred early in life. However, 7 of the 90 patients (8%) presented with late-onset lymphoproliferation during adulthood. Few cases with late onset have been reported in the literature.27 Although these observations are rare, they emphasize the need to consider a diagnosis of ALPS in adulthood. Autoimmune manifestations were also frequent and usually occurred a few years after lymphoproliferation. Cytopenia was the most frequently observed sign, as reported previously. The pattern of autoimmunity in ALPS patients differs from that seen in lpr mice.28-30 Depending on the genetic background, this animal model mainly develops lupus-like autoimmune disease and nephritis. In ALPS patients, cytopenia is far more common. Glomerulonephritis occurred in only 3% of our cohort and no cases of systemic lupus erythematosus were observed.
In all patients monitored over a long period of time, lymphoproliferation decreased or disappeared at adulthood. This observation contrasts with the situation in lpr mice, in which DN T cells accumulate over time and the lifespan is shortened.28 Active thymopoiesis and antigenic stimulation in early childhood may increase the T-lymphocyte load in the periphery (particularly for the CD8+ thymocytes that give rise to DN T cells).31 Another possible explanation is the progressive involvement of an alternative, compensatory Fas-independent apoptotic mechanism. In contrast, more than one-half of the adult patients continued to present autoimmune complications in general and autoimmune cytopenia in particular. It may be that self-reactive B-cell clones give rise to long-lasting plasma cells capable of producing autoantibodies for a very long period of time. The contrast between the remission of lymphoproliferation and the persistence of autoimmunity suggests that lymphoproliferation is not simply a consequence of the accumulation of self-reactive clones.
Within the patient group, we observed a strikingly high incidence of severe, postsplenectomy infections (mainly due to S pneumoniae), but no other susceptibility to infections. The increased risk of overwhelming, postsplenectomy infections has already been noted by Canale and Smith and others,1,32 but our report is the first to have assessed this factor as the main cause of death in our cohort. Thirty percent of the splenectomized patients had at least 1 episode of severe infection (> 20 years after splenectomy in some individuals). Although this risk has been well documented in asplenic patients and splenectomized individuals, the incidence observed here is much higher than expected.33 Despite identified risk factors (young age at splenectomy and poor adherence to prophylactic measures), the incidence of severe postsplenectomy infections is high. The reason for this increased susceptibility is unknown and is not clearly related to addition of immunosuppressive treatment. In any event, splenectomy should be avoided in ALPS patients unless absolutely essential.
In our experience, 64 patients (86%) with ALPS required treatment. Autoimmune manifestations, in particular autoimmune cytopenia, required immunosuppressive treatment in most cases. It is unclear whether ALPS patients with autoimmunity should be treated in a similar manner as patients with the same autoimmune manifestation in the absence of ALPS. The use of immunosuppressive drugs with proapoptotic and/or antiproliferative properties (eg, azathioprine, MMF, 6MP, and rapamycin) could be preferentially used based on disease pathophysiology. Toxicity has to be considered given the fairly high risk of recurrence. Anti-CD20 antibody therapy in ALPS patients does not appear to be—at least for AIHA—as effective as in patients without ALPS.24
It is already known that ALPS is accompanied by an increased risk of lymphoma.2,25,34 Thirteen cases of various lymphoid malignancies (mainly B-cell NHL and HL, including nodular lymphocyte–predominant HL) were reported in 12 patients. The relative risks of developing HL and NHL were estimated to be 51 and 14, respectively.2 In previous reports, all affected patients carried TNFRSF6 mutations within the ICD and particularly within the DD. In our cohort, the cumulative risk of developing lymphoid malignancy before the age of 30 years was calculated to be 15%. Some affected patients carried ECD and mosaic mutations rather than germline TNFRSF6 ICD mutations, extending the spectrum of “at-risk” patients. Glioma has been reported previously in 1 ALPS patient.18 Therefore, this association between 2 rare conditions may not have occurred by chance.
In the present study, the penetrance of ECD and ICD mutants in relatives was 28% and 40%, respectively, with a higher penetrance (56%) for missense mutations inside the DD; for carriers as a whole, the penetrance values were 52%, 63%, and 73% for ECD mutations, ICD mutations, and DD missense mutations, respectively. The observed penetrance rate for ECD mutations agrees with previous studies.18,22,35 It is noteworthy that the penetrance of DD mutants observed here is much lower than the value of 85%-90% reported previously in relatives with DD missense mutations.18,22,35 We cannot strictly rule out the possibility that our retrospective analysis resulted in an underestimation of disease-related symptoms in relatives. In contrast, half of the mutations reported here are new. Although a potential DN effect has not been studied for the latter, one can speculate that several lead to haploinsufficiency that is associated with lower penetrance.
The variable clinical penetrance of TNFRSF6 mutations and the differing phenotype found in patients from the same family indicate that additional genetic or environmental factors determine ALPS. Indeed, we recently reported the additional presence of a somatic event impairing the second TNFRSF6 allele (ie, somatic mutation or duplication of the mutant allele with loss of the wild-type allele) in 7 patients with germline ECD mutation.19 Five of these patients were included in this survey and 4 additional patients were identified. Co-mutation was frequently found in ALPS-FAS patients carrying ECD mutations (8 of 12, 66%). The latter are known to induce loss of Fas expression at the cell membrane and therefore produce haploinsufficiency,19 the second mutation leading to loss of membrane FAS expression. Similar events were screened for in selected patients carrying ICD mutants with low clinical penetrance, but were rarely identified (1 of 6, 17%). This latter patient carried a germline frameshift mutation at the end of exon 9 with somatic telomeric uniparental disomy and displayed a complete Fas expression defect in DN T cells. Interestingly, germline mutation patients with somatic changes in the second TNFRSF6 allele were significantly older at disease onset than other ALPS-FAS or ALPS-sFAS patients, suggesting that the second “hit” may be acquired later in life. Overall, the absence of genotype-phenotype correlation suggests that other factors dictate the occurrence of autoimmunity and the disease severity. Phenotypic heterogeneity is also observed in mice carrying the TNFRSF6 mutation, with the autoimmune manifestations varying as a function of the genetic background.28
We observed significant male predominance among index cases and symptomatic relatives and female predominance in asymptomatic relatives. These gender ratios did not appear to depend on the genotype characteristics, although the number of ALPS-sFAS patients was small. Interestingly, Dowdell et al10 reported a series of 12 ALPS-sFAS patients with a male/female gender ratio of 3. Bleesing et al36 found a male/female gender ratio of 1.8 in ALPS-FAS probands. This observation contrasts with the female predominance found in many autoimmune diseases37 and suggests the involvement of a predisposing genetic factor on the Y chromosome or a recessive X-linked factor.
This study of a large cohort of ALPS-FAS and ALPS-sFAS patients highlighted the significant morbidity of this disease, which was mainly related to long-term autoimmune manifestations and an increased risk of lymphoma. It also emphasized the high risk of postsplenectomy invasive bacterial infections and related mortality, which might suggest the existence of specific susceptibility in this patient population. Further prospective studies are needed to determine the risk factors for developing ALPS symptoms and thus the appropriate treatment for individual patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the clinicians who cared for the patients (Dr Anne Auvrignon, Dr Francoise Bernaudin, Pr Jean-Laurent Casanova, Dr M. Debré, Dr J. J. Debruycker, Pr Isabelle Durieu, Pr Alice Ferster, Dr F. Haerynck, Dr Thierry Leblanc, Dr G. Laureys, Dr Margueritte Micheau, Dr Isabelle Thuret, Dr Valérie Mialou, Pr Yves Perel, Dr Pascal Pillet, Dr F. Ries, Dr Rivière, Dr Caroline Thomas, and Dr Christel Van Geet); the patients and their families; Corinne Jacques, Chantal Harre, Stéphanie N'Daga, and Aminate Diabate for excellent technical assistance; and CEREVANCE (the French reference center for autoimmune cytopenia).
This work was supported by grants from Inserm (to B.N. and N.L.); the Fondation pour la Recherche Médicale (to F.R.-L. and B.N.); the Agence Nationale pour la Recherche (to F.R.-L.); and by an advanced senior grant from the European Research Council (to A.F.).
Authorship
Contribution: B.N. designed the research, collected and analyzed the data, and participated in writing the manuscript and in the clinical care of the patients; A.M.-C. performed genetic and biologic diagnosis of the patients, analyzed the data, and critically read the manuscript; B.F., D.G., O.L., and L.D.S. collected and analyzed the data, participated in the clinical care of the patients, and critically read the manuscript; N.L. and M.-C.S. participated in the biologic diagnosis of the patients; B.B.-M, N.A., C.C, Y.B., E.J., G.L., G.M., F.S., E.O., O.H., and S.B. participated in the clinical care of the patients; C.P. participated in the genetic and biologic diagnosis of the patients and in the clinical care of the patients; A.F. participated in analysis of the data, writing of the manuscript, and the clinical care of the patients; and F.R.-L. participated in the genetic and biologic diagnosis of the patients, analysis of the data, and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bénédicte Neven, Inserm U768, Hôpital Necker-Enfants Malades, 149 Rue de Sèvres, F-75015 Paris, France; e-mail: benedicte.neven@nck.aphp.fr.
References
Author notes
A.M.-C. and B.F. contributed equally to this work.
A.F. and F.R.-L. share senior authorship.

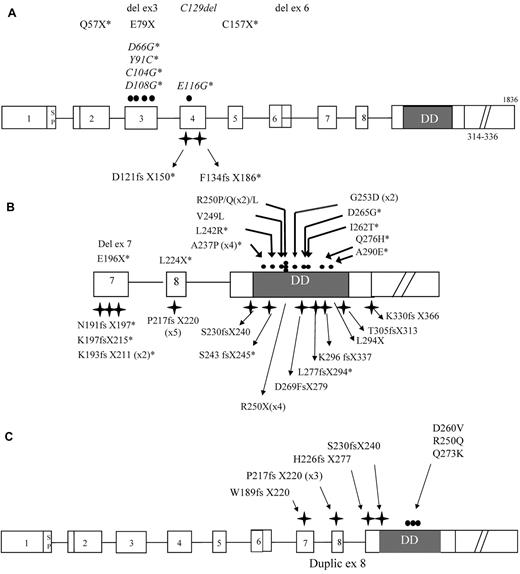

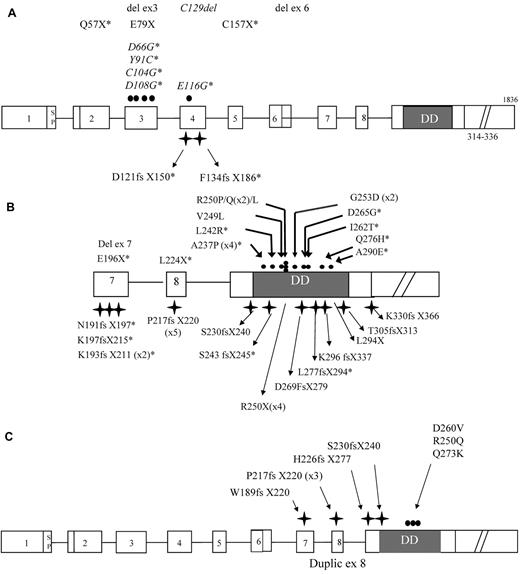
 ); deletions and nonsense mutations are also indicated. Mutations denoted with an asterisk are novel. The numbers of unrelated index cases carrying the same mutation are given in brackets. Mutations associated with ALPS-sFAS are shown in panel C.
); deletions and nonsense mutations are also indicated. Mutations denoted with an asterisk are novel. The numbers of unrelated index cases carrying the same mutation are given in brackets. Mutations associated with ALPS-sFAS are shown in panel C.