Abstract
Thymus atrophy is the most common immunopathology in humans, and its occurrence is hastened by several factors that coalesce in patients receiving chemotherapy and most of all in recipients of hematopoietic cell transplantation. We have shown previously that posthematopoietic cell transplantation thymic function was improved by retroviral overexpression of Wnt4 in donor hematopoietic cells. Here, by using both conventional and conditional null mutant mice, we show that Wnt4 regulates steady-state thymic cellularity by a thymic epithelial cell (TEC)–dependent mechanism. The absence of Wnt4 suppressed fetal and postnatal thymic expansion and resulted in decreased TEC numbers, an alteration of the medullary-to-cortical TEC ratio, and a disproportionate loss of the most immature cKithi thymocyte precursors. Wnt4 also is implicated in the maintenance of adult thymopoiesis, although the impact of its deletion once thymic involution has been initiated is more subtle. Together, our results show that Wnt4 controls thymic size by modulating TEC expansion and the earliest, TEC-dependent steps of thymocyte development both in the fetal and postnatal thymus. Wnt4 and its downstream signaling pathways could thus represent interesting candidates to improve thymic output in subjects with thymic atrophy.
Introduction
T lymphopoiesis is a complex and highly regulated process that involves multiple dynamic interactions of the developing thymocyte with its microenvironment. Although vertebrate hematopoiesis is known to occur in a variety of locations, the thymus is the unique site for T-lymphocyte differentiation and repertoire selection in vertebrates.1 Thymus atrophy ultimately affects all ageing subjects and contributes to the age-related increase in the incidence of infections and cancer.2 Thymic failure also can be accelerated by various stressors that may affect subjects of any age: chemotherapy, corticosteroids, disturbed lipid metabolism, infection, inflammation, limiting numbers of thymus-seeding progenitor cells, and psychologic stress.2-5 These factors coalesce in recipients of hematopoietic cell transplantation (HCT) who therefore suffer from life-threatening thymic failure for protracted periods.6-8
Thymus organogenesis and the maintenance of thymus architecture are dependent on lymphostromal cross talk.9,10 Although thymic epithelial cells (TECs) provide growth factors and signals that will promote migration and T lineage commitment to the developing lymphocytes, the presence of lymphocytes is also necessary for the differentiation and functional maturation of TECs.11,12 Postnatal disruption of TECs results in thymic atrophy,13,14 whereas restoration of thymocyte development can reestablish TEC architecture in cases where the defect was thymocyte intrinsic.15,16
Several studies have shown the importance of proper regulation of Wnt signaling during T-cell development.17,18 The majority have focused on the importance of canonical Wnt signals in the developing T lymphocytes, whereas the possible impact of Wnt signals on the thymic stroma has been less studied. Inducible overexpression of the Wnt inhibitor Dkk1 in TECs resulted in the loss of keratin (K)8+K5+ (progenitor) TECs and reversible thymic involution.14 Conversely, the Wnt antagonist Kremen was shown to be necessary for the establishment of normal TEC numbers during thymic organogenesis.19 Interestingly, stabilization of β-catenin in epithelial cells prevented thymic development,20,21 despite the fact that canonical Wnt signaling, and in particular Wnt4, have been suggested to induce FoxN1 expression.22 However, none of these studies have focused on the effect of an individual Wnt family protein nor have they considered the potential impact of noncanonical Wnt signaling. Nevertheless, based on these studies, down-regulation of the canonical Wnt signaling pathway seems necessary at least at some point during thymic organogenesis.
We have reported previously that overexpression of Wnt4 in donor hematopoietic cells improved thymic recovery after hematopoietic cell transplantation (HCT).23 Under conditions of hematopoietic stress (fetal hematopoiesis and post-HCT),24 the effect of Wnt4 was at least in part prethymic: Wnt4 expands bone marrow and fetal liver hematopoietic progenitors through a planar cell polarity-like pathway involving Fz6, Rac1, and JNK kinases.25 The goal of our present study was to examine the intrathymic role of Wnt4 in fetal and postnatal thymopoiesis using both conventional and conditional Wnt4 null mutant mice. We report that Wnt4 was necessary for normal thymocyte and TEC numbers, as well as for the establishment of a proper medulla during fetal thymopoiesis and postnatal thymic expansion. We also show that Wnt4 was implicated in the first TEC-dependent steps of thymocyte expansion, because the proportion of early thymic progenitors (ETPs; Lin−cKithiCD25−) and double-negative (DN) 2 thymocytes (Lin−cKithiCD25+) was decreased in the absence of Wnt4. The impact of Wnt4 on ETPs and DN2 thymocytes was probably direct because it could be reproduced in the OP9-DL1 coculture system. Together, our results show that Wnt4 is important for the establishment of the thymic microenvironment and is still active during steady-state postnatal thymopoiesis in regulating the expansion of intrathymic T-cell precursors as well as the epithelial compartment.
Methods
Mice
C57BL/6 (B6; CD45.2+), B6.SJL-PtprcaPep3b/BoyJ (Ly5a; B6.SJL; CD45.1+), B6.Cg-Tg(tetO-Cre)1Jaw/J (tetO-Cre), B6.Cg-Gt(ROSA)26Sortm1(rtTA*M2)Jae/J (ROSArtTA), and B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (ROSAeYFP) mice were purchased from The Jackson Laboratory. Tcf7−/− mice26 were provided by Dr H. Clevers (Hubrecht Institute, Utrecht, The Netherlands). Wnt4 conventional knockout27 and Wnt4lox/lox mice28 have been described previously. To induce Wnt4 deletion in tetO-Cre ROSArtTA/+Wnt4lox/lox mice, 2 different protocols were used (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To delete Wnt4 at birth, pregnant females that lacked the Cre transgene were given doxycycline (2 mg/mL in drinking water supplemented with 5% sucrose) beginning at 18.5 days p.c., and the females were maintained on doxycycline until the pups were weaned. The mice were then kept off doxycycline for 4 weeks before analysis. To delete Wnt4 in the adult, 5- to 6-week-old mice were given 4 intraperitoneal injections of doxycycline in PBS (80 μg/g body weight) and analyzed at 4 or 8 weeks after the first injection. All mice were bred and housed under specific-pathogen-free conditions in sterile ventilated racks at the Institute for Research in Immunology and Cancer (IRIC), Université de Montréal. All procedures were in accordance with the Canadian Council on Animal Care guidelines and approved by the Comité de Déontologie et Expérimentation Animale de l'Université de Montréal.
Analysis of proliferation and apoptosis for thymocyte subsets
Engrafted mice were injected intraperitoneally with 1 mg of BrdU in PBS to provide a labeling pulse. Four hours later, thymic grafts were harvested, and BrdU uptake of thymocytes was detected with a BrdU flow kit (BD Biosciences Pharmingen) following the manufacturer's instructions. For the annexin V apoptosis assay, thymocytes were labeled with cell surface markers and then stained with Alexa Fluor 350–conjugated antibody to annexin V (Invitrogen). Propidium iodide was used to exclude dead cells from the apoptosis assays.
Analysis of proliferation and apoptosis of TECs
Embryonic day (E)15.5 thymic lobes were isolated and placed in fetal thymic organ culture as described previously.11 BrdU was added to the medium at a final concentration of 10μM for an overnight incubation. The lobes were then harvested and dissociated using trypsin. Before BrdU analysis, cells were labeled with cell surface markers. BrdU uptake of TECs was detected with a BrdU flow kit (BD Biosciences Pharmingen) following the manufacturer's instructions. For apoptosis of TECs, E15.5 thymi were isolated, labeled with cell surface markers, and stained with an In Situ Cell Death Detection kit (Roche Diagnostics) following the manufacturer's instructions.
Thymus homing
The thymus homing assay was adapted from a previously described protocol.29 In brief, 30 × 106 nucleated BM cells from congenic (CD45.1+) mice were injected into the lateral tail vein of doxycycline-treated, nonirradiated adult mice (CD45.2+). Thymi were harvested 36 hours later, and 5 × 106 thymocytes were labeled for flow cytometry analysis.
OP9-DL1 coculture
The generation of OP9-DL1 cells has been described previously.30 To overexpress Wnt4 in the OP9-DL1 system, the cells were transfected with the pUSE-Amp-Wnt4 plasmid (Millipore) and selected for 10 days with 400 μg/mL geneticin (Invitrogen). Expression of Wnt4 was confirmed by Western blot. Sorted ETPs from young C57BL/6 females were seeded on 80% to 90% confluent monolayers and cultured in the presence of 2 ng/mL recombinant IL-7 (PeproTech) and 5 ng/mL recombinant Flt3L (PeproTech) for indicated times.
Immunohistology
Thymi were harvested from embryos at stage E18.5, fixed in 10% formalin, and mounted in paraffin. Sections were stained with H&E, and central sections were scanned using the NanoZoomer Digital Pathology system and NPD.scan 2.3.4 software (Hamamatsu). Additional information is found in supplemental Methods.
RNA extraction and qRT-PCR
RNA was extracted with TRIzol reagent (Invitrogen) and was further cleaned up with the RNeasy microkit columns (QIAGEN) following the manufacturers' instructions. Reverse transcription was carried out using High Capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative (q)RT-PCR was performed using a 7900HT Fast Real-Time PCR system (Applied Biosystems) at the Institute for Research in Immunology and Cancer's genomic core facility. The relative quantification of target genes was determined by using the ΔΔCT method.
Thymic stromal cell enrichment
This procedure was adapted from a previously described protocol.31 In brief, thymi were isolated, and thymocytes were removed by gently crushing the organ in between 2 microslides. The tissue was then stirred for 5 minutes at 4°C in serum-free RPMI-1640 to free more thymocytes. Remaining fragments were dissociated by a series of enzymatic digestions using 0.36mM CaCl2, 0.125% (wt/vol) collagenase IV (Sigma-Aldrich), and 0.1% (wt/vol) DNAse I (Sigma-Aldrich) in RPMI-1640. Supernatants were collected, centrifuged, and resuspended in EDTA/FACS buffer (5mM EDTA in PBS with 1% FBS). Cells were stained with cell surface markers and subsequently analyzed by flow cytometry.
Thymic grafts
A small dorsolateral incision was made to expose the kidney, and a small hole was cut into the kidney capsule of anesthetized adult C57BL/6 recipients. E18.5 thymic lobes from Wnt4+/+ and Wnt4−/− mice were grafted under the kidney capsule of recipient mice. The incision was closed using surgical sutures. Four weeks after transplantation, the grafts were removed and analyzed by flow cytometry or immunohistochemistry.
Thymic aggregates
Thymic aggregates were obtained after a compaction protocol described previously.32 In brief, thymi were harvested from Wnt4−/− embryos at E15.5. Thymic lobes were then dissociated into a single-cell suspension and mixed with 2 × 105 NIH3T3 cells expressing either green fluorescent protein (GFP) or Wnt4-internal ribosomal entry sequence-GFP. The mixture was aspirated into a nonbeveled pipette tip sealed with folded parafilm and centrifuged at 300g for 5 minutes. Aggregates were incubated at 37°C for 5 days on a floating 0.8-mm Isopore membrane filter (Millipore).
Statistical analysis
Two-tailed Student t test was used to determine statistical significance. P values of .05 or less are considered significant.
Results
Wnt4 is predominantly expressed by the TECs
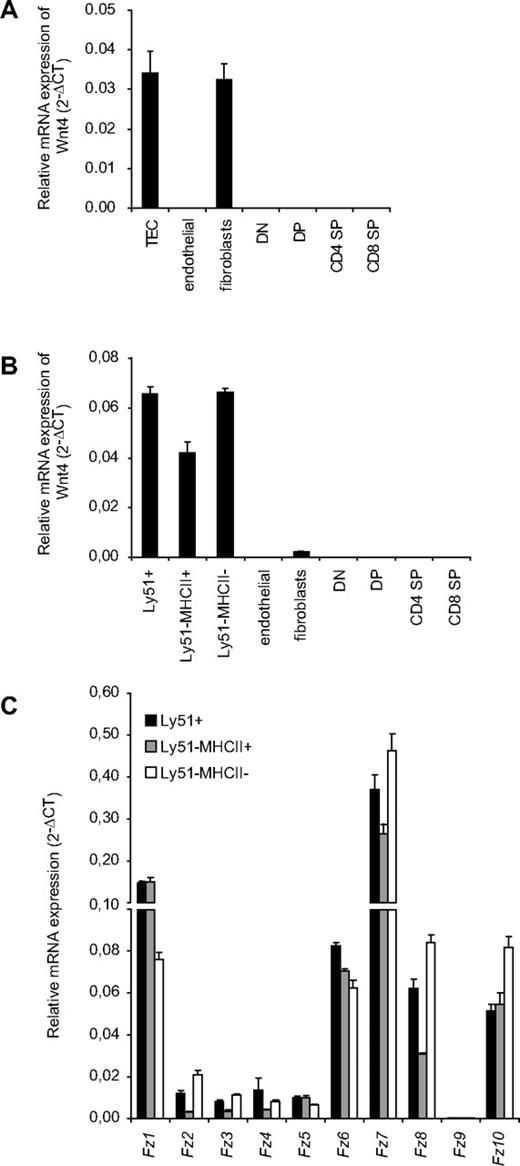
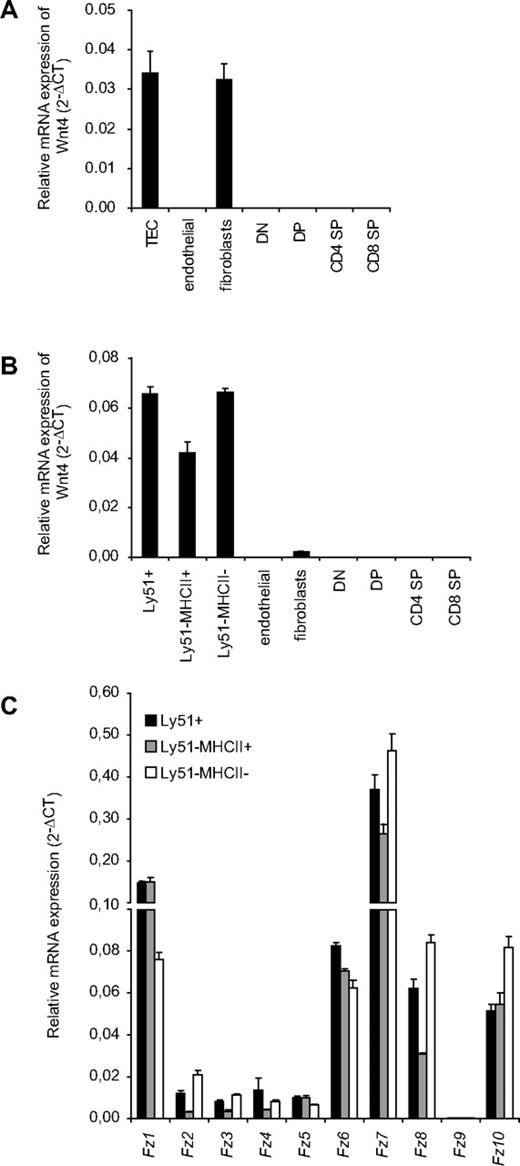
Wnt4 has been shown previously to be expressed in fetal TECs,22,33 but little is known on its potential expression by other thymic stromal components and thymocyte subsets. We now compared Wnt4 mRNA expression by qRT-PCR in different thymic stromal fractions (TECs, endothelial cells, and fibroblasts) as well as thymocytes both in E18.5 fetal thymus and in young adult mice. In the fetal thymus, Wnt4 was strongly expressed in the EpCAM+ TECs as well as in fibroblasts (Figure 1A). In the adult mice, however, Wnt4 was predominantly expressed by both Ly51+ cortical (c)TECs and Ly51− medullary (m)TECs (Figure 1B), with only low levels in fibroblasts and no detectable transcripts in either endothelial cells or thymocytes. The predominant Frizzled transcripts Fz1 and Fz7 also were detected in all TEC subsets (Figure 1C). These results show that Wnt4 expression in the thymus is limited to stromal cells and is practically TEC-restricted in the adult. In addition, TECs express a variety of different Frizzled receptors that can convey Wnt signals.
Wnt4 is predominantly expressed byTECs. Relative mRNA expression for Wnt4 in total TECs (CD45−EpCAM+), cTECs (Ly51+), immature mTECs (Ly51−MHCII−), mature mTECs (Ly51−MHCII+), endothelial cells (CD45−CD31+), fibroblasts (CD45−EpCAM−Ly51+), and in T-cell subsets (DN, DP, CD4 SP, and CD8 SP) of E18.5 (A) and 4-week-old adult (B) mice, expressed as mean 2−ΔCT standardized to the endogenous control GAPDH. (C) Relative mRNA expression of Fz receptors in adult TEC subsets is expressed as mean 2−ΔCT standardized to the endogenous control GAPDH. Histogram represents mean ± SEM of 3 mice.
Wnt4 is predominantly expressed byTECs. Relative mRNA expression for Wnt4 in total TECs (CD45−EpCAM+), cTECs (Ly51+), immature mTECs (Ly51−MHCII−), mature mTECs (Ly51−MHCII+), endothelial cells (CD45−CD31+), fibroblasts (CD45−EpCAM−Ly51+), and in T-cell subsets (DN, DP, CD4 SP, and CD8 SP) of E18.5 (A) and 4-week-old adult (B) mice, expressed as mean 2−ΔCT standardized to the endogenous control GAPDH. (C) Relative mRNA expression of Fz receptors in adult TEC subsets is expressed as mean 2−ΔCT standardized to the endogenous control GAPDH. Histogram represents mean ± SEM of 3 mice.
Loss of Wnt4 results in a decreased frequency of fetal and postnatal ETPs
It has been suggested that Wnt proteins in general provide growth signals to the developing thymocytes.33 Wnt4 in particular has been shown to regulate fetal thymocyte numbers with little effect on thymocyte differentiation.34,35 Furthermore, we have shown previously that Wnt4 can improve thymic recovery in the adult after irradiation and BM transplantation,23 mainly through the expansion of ETP and DN2 subsets in the thymus and lymphoid-primed multipotent progenitors in the bone marrow. We now wanted to further examine the impact of Wnt4 on the most immature thymocyte populations at different stages of thymopoiesis: in the fetal thymus, during the postneonatal thymic expansion, and in the adult thymus.
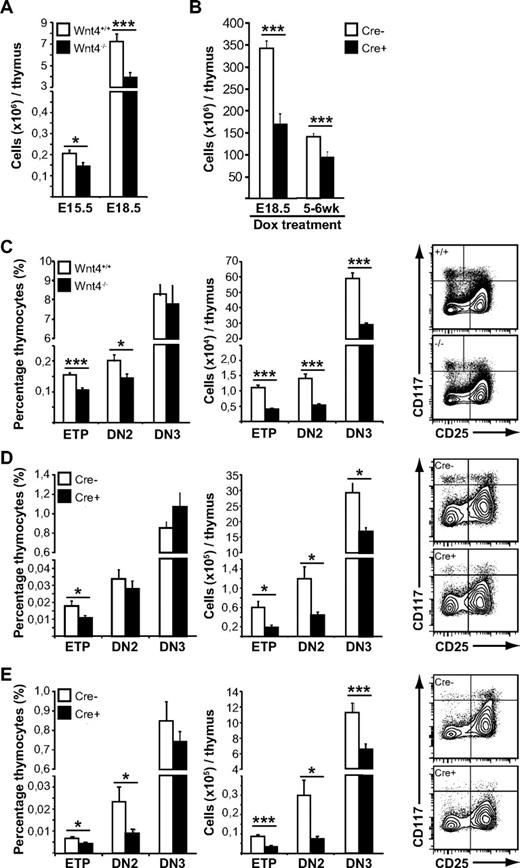
As expected, the lack of Wnt4 in the fetal thymus resulted in decreased cellularity (Figure 2A). There was also a disproportionate decrease in the percentages of ETPs and DN2 in the Wnt4−/− thymus (Figure 2C left panel). The numbers of DN3, immature single-positive (SP) and double-positive (DP) thymocytes also were decreased (Figure 2C; supplemental Figure 2A), but their relative frequencies were not.
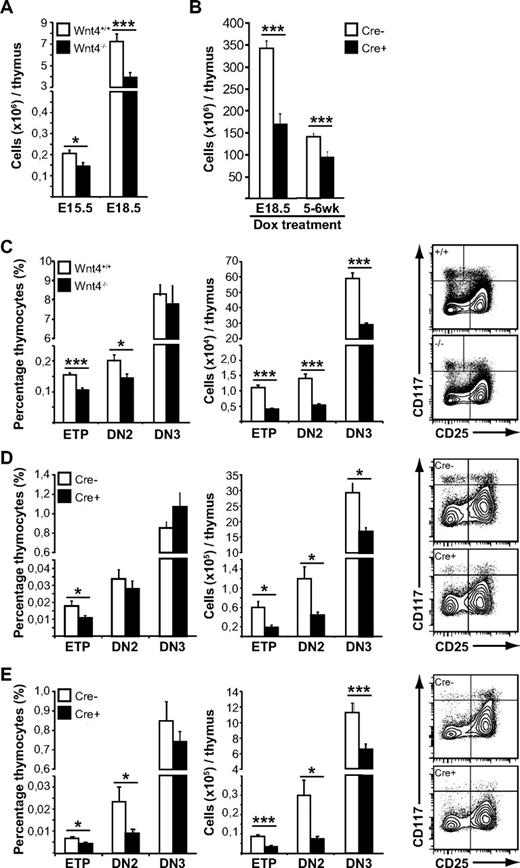
Wnt4 is needed for steady-state postnatal thymopoiesis. (A) Thymic cellularity from E15.5 and E18.5 Wnt4+/+ and Wnt4−/− mice. (B) Thymic cellularity from mice in which Wnt4 deletion has been induced (Cre+) on E18.5 or in the adult, compared with Cre− littermate controls. (C-E) FACS analysis of lineage-negative thymocytes from E18.5 Wnt4+/+ and Wnt4−/− mice (C), mice in which Wnt4 has been deleted at birth (D), and mice in which Wnt4 has been deleted in adulthood (E). The graphs on the left show the proportion of ETP (Lin−cKithiCD25−), DN2 (Lin−cKithiCD25+), and DN3 (Lin−cKit−/loCD25+) subsets expressed as percentage of total thymocytes. The graphs in the middle show the absolute numbers of cells within each subset per thymus. Representative flow cytometry data are shown on the right. All histograms represent the mean ± SEM (n = 6-7/group; *P < .05, **P < .01, ***P < .005).
Wnt4 is needed for steady-state postnatal thymopoiesis. (A) Thymic cellularity from E15.5 and E18.5 Wnt4+/+ and Wnt4−/− mice. (B) Thymic cellularity from mice in which Wnt4 deletion has been induced (Cre+) on E18.5 or in the adult, compared with Cre− littermate controls. (C-E) FACS analysis of lineage-negative thymocytes from E18.5 Wnt4+/+ and Wnt4−/− mice (C), mice in which Wnt4 has been deleted at birth (D), and mice in which Wnt4 has been deleted in adulthood (E). The graphs on the left show the proportion of ETP (Lin−cKithiCD25−), DN2 (Lin−cKithiCD25+), and DN3 (Lin−cKit−/loCD25+) subsets expressed as percentage of total thymocytes. The graphs in the middle show the absolute numbers of cells within each subset per thymus. Representative flow cytometry data are shown on the right. All histograms represent the mean ± SEM (n = 6-7/group; *P < .05, **P < .01, ***P < .005).
To address the physiologic role of Wnt4 in early postnatal thymopoiesis, we generated mice in which Wnt4 deletion in all cells can be induced by doxycycline (supplementary Figure 1).28 Given that Wnt4 is expressed in the fetal kidney and is required for early renal development,27 we started doxycycline treatment of pregnant females that lacked the Cre transgene at 18.5 days p.c. (when kidney development is completed), and we maintained the females on doxycycline until the pups were weaned. Thus, Wnt4 was deleted immediately before birth, allowing for normal thymic organogenesis to take place but interfering with the first wave of postnatal thymopoiesis (supplemental Figure 1).
Thymi from doxycycline-treated tetO-Cre ROSArtTA/+Wnt4lox/lox mice (Cre+) analyzed at 7 to 8 weeks of age were hypocellular compared with littermate controls that lacked the Cre-transgene (Cre−; Figure 2B), thus demonstrating that Wnt4 expression was still necessary in the postnatal thymus. Analysis of the thymocyte compartment revealed no major block in T-cell differentiation (supplemental Figure 2B). There was a disproportionate decrease in the most immature T-cell precursors (ETPs; Figure 2D), but all T lineage–committed cells were present at normal proportions, albeit at lower numbers.
Deletion of Wnt4 at birth thus suppressed the early postnatal thymic expansion phase. Next, we wanted to determine whether Wnt4 also was required for the maintenance of adult thymopoiesis. Young adult mice (5-6 weeks old) were given 4 intraperitoneal injections of doxycycline and then analyzed 8 weeks after the first injection. Thymi from tetO-Cre ROSArtTA/+Wnt4lox/lox mice displayed a 30% decrease in total cellularity (Figure 2B), once more no major block in differentiation (supplemental Figure 2C), but rather a disproportionate decrease in both ETPs and the highly proliferative DN2 subset (Figure 2E). Together, our results at 3 different stages of thymopoiesis indicate that Wnt4 plays a physiologic role in the regulation of thymic size but not differentiation past the DN2 stage in the fetal, neonatal, and young adult mouse. Most importantly, we demonstrate that Wnt4 is still active during steady-state postnatal and adult thymopoiesis.
Wnt4 directly enhances ETP and DN2 expansion
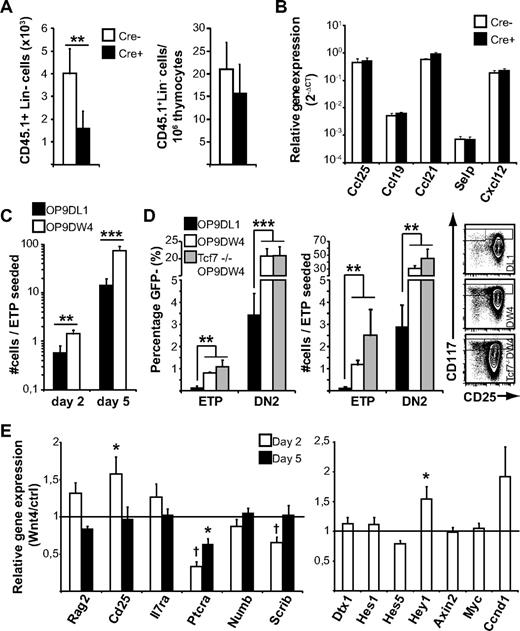
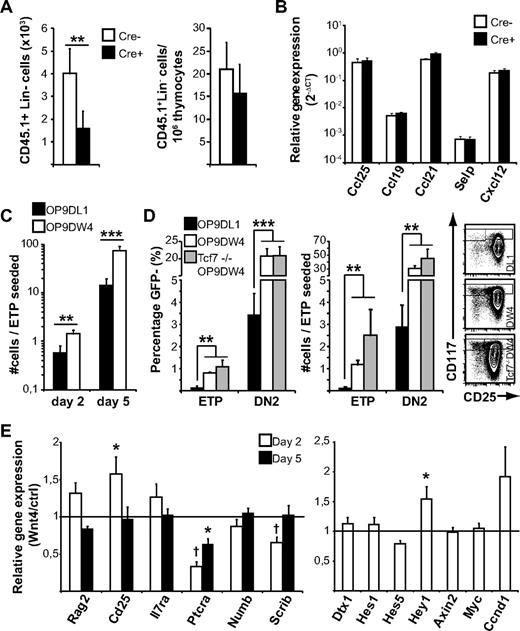
The concept of Wnt4 regulating only the most immature thymocytes has been alluded to previously23 ; however, the mechanism, particularly in the adult, is not clear. Our previous reports suggest that the effect of Wnt4 on bone marrow progenitors might contribute to the increase in ETPs in Wnt4 overexpression chimeras.23,25 However, we did not detect any notable bone marrow progenitor defect in our doxycycline-treated tetO-Cre ROSArtTA/+Wnt4lox/lox mice (data not shown), suggesting that the decrease in the proportion of ETPs would not be because of a prethymic effect. To further confirm this hypothesis, we injected wild-type congenic bone marrow into nonirradiated doxycycline-treated mice and harvested the thymi after 36 hours. There was a 2-fold decrease in the total number of lineage-negative donor cells recovered from tetO-Cre ROSArtTA/+Wnt4lox/lox thymi compared with Cre− littermate controls (Figure 3A left panel). In addition, the proportion of donor cells in the thymus was similar between Cre+ and Cre− mice (Figure 3A right panel), indicating that the ETP defect was noncell autonomous and not because of a defect of the endogenous precursors from Wnt4-deficient hosts. We detected no difference in the expression of P-selectin and key chemokines between Cre+ and Cre− thymi (Figure 3B), suggesting that there was no major problem with thymic import but that the decrease in thymocyte progenitors might be because of impaired expansion in situ.
Wnt4 has a direct effect on ETP and DN2 expansion. (A) Analysis of homing to the Wnt4-deficient and -sufficient adult thymus. The number and relative contribution of donor-derived (CD45.1+) Lin− cells was determined 36 hours after injection. The histograms represent mean ± SEM from 7 mice per group. (B) Expression of chemokines and P-selectin in the Wnt4-deficient and -sufficient adult thymus. The histograms represent mean ± SEM from 4 to 5 mice per group. (C) Analysis of T-cell development in culture in the presence of GFP+ OP9-DW4 or OP9-DL1 stromal cells. GFP− cells were analyzed by flow cytometry at indicated times and are expressed as number of cells/ETP seeded on day 0. The histogram represents mean ± SEM from a total of 8 replicates from 3 independent experiments. (D) Analysis of T-cell differentiation as defined by cKit and CD25 staining on day 5. The histogram on the left represents ETP and DN2 frequencies among GFP− cells. The histogram in the middle represents the number of cells with ETP and DN2 phenotype on d5 per ETP seeded (mean ± SEM; n = 8 for wild type, n = 5 for Tcf7−/−). Representative flow cytometry data gated on GFP− cells are shown on the right. (E) Expression of selected genes associated with DN2-DN3 progression and early T-cell development. Histogram represents the relative mRNA levels from sorted GFP− lymphocytes after 2 or 5 days of coculture (ratio OP9-DW4/OP9-DL1 from paired experiments; mean ± SEM; n = 3; *P < .05, **P < .01, ***P < .005).
Wnt4 has a direct effect on ETP and DN2 expansion. (A) Analysis of homing to the Wnt4-deficient and -sufficient adult thymus. The number and relative contribution of donor-derived (CD45.1+) Lin− cells was determined 36 hours after injection. The histograms represent mean ± SEM from 7 mice per group. (B) Expression of chemokines and P-selectin in the Wnt4-deficient and -sufficient adult thymus. The histograms represent mean ± SEM from 4 to 5 mice per group. (C) Analysis of T-cell development in culture in the presence of GFP+ OP9-DW4 or OP9-DL1 stromal cells. GFP− cells were analyzed by flow cytometry at indicated times and are expressed as number of cells/ETP seeded on day 0. The histogram represents mean ± SEM from a total of 8 replicates from 3 independent experiments. (D) Analysis of T-cell differentiation as defined by cKit and CD25 staining on day 5. The histogram on the left represents ETP and DN2 frequencies among GFP− cells. The histogram in the middle represents the number of cells with ETP and DN2 phenotype on d5 per ETP seeded (mean ± SEM; n = 8 for wild type, n = 5 for Tcf7−/−). Representative flow cytometry data gated on GFP− cells are shown on the right. (E) Expression of selected genes associated with DN2-DN3 progression and early T-cell development. Histogram represents the relative mRNA levels from sorted GFP− lymphocytes after 2 or 5 days of coculture (ratio OP9-DW4/OP9-DL1 from paired experiments; mean ± SEM; n = 3; *P < .05, **P < .01, ***P < .005).
Differential recovery of wild-type lineage-negative cells in the thymus of Wnt4-deficient versus -replete hosts (Figure 3A left panel) could be because of an effect of Wnt4 on the thymus stroma, thymus seeding progenitors, or a combination. To determine whether Wnt4 might act directly on thymus seeding progenitors in contrast to a strictly stromal effect, we seeded sorted wild-type ETPs either on OP9-DL1 stromal cells or on OP9-DL1 cells that were stably transfected to overexpress Wnt4 (OP9-DW4), and then we compared their expansion and differentiation during coculture. Stromal Wnt4 had no apparent effect on the ETP→DN2 transition, because the percentage of CD25+ cells was comparable in both cultures on day 2 (data not shown). However, already after 48 hours of coculture, the number of cells in Wnt4+ cultures was increased by 2- to 3-fold (Figure 3C). Interestingly, the presence of Wnt4 caused the retention of a more immature phenotype, because the proportion of ETPs and DN2s was significantly higher in OP9-DW4 cultures than on OP9-DL1 on day 5 (Figure 3D left panel). In fact, the number of ETPs remaining in OP9-DW4 cultures was at least comparable to the number of ETPs seeded and the proportion of DN2 cells was 6 times higher in the presence of exogenous Wnt4 (Figure 3D middle panel). In comparison, there were only twice as many DN3 cells in OP9-DW4 cultures compared with OP9-DL1 (245 ± 42 DN3s/ETP seeded for OP9-DW4 vs 132 ± 49 for OP9-DL1; P < .01). Together, these results suggest that Wnt4 can exert a direct, paracrine influence on ETPs and DN2s, independent of possible prethymic effects in the bone marrow or potential indirect effects on the thymic microenvironment.
To further examine the potential mechanism of action of Wnt4, we sorted the GFP− lymphocyte fraction from 2- and 5-day cocultures and analyzed the expression of selected target genes by quantitative RT-PCR. In accordance with previous results,25 we saw a slight but transient increase in the expression of Cd25 (Figure 3E) as well as the Hes-related Notch target gene Hey1. However, the canonical Notch target genes (Dtx, Hes1, and Hes5) were expressed at similar levels in both groups, indicating that Wnt4 did not enhance general Notch activation.36 In contrast, the expression of Ptcra and the polarity complex gene Scrib was decreased in the presence of Wnt4. Scribble inhibition in fetal liver progenitor cells results in a partial block during DN3 progression.37 Down-regulation of Ptcra and Scrib correlates with the delayed DN2→DN3 progression and could contribute to the increased proportion of ETP/DN2 thymocytes observed in the presence of Wnt4 in vivo and in vitro. In various cell types, Wnt4 can elicit canonical, noncanonical, or both types of Wnt signaling. We reported previously that Wnt4 induces only noncanonical signals in bone marrow and fetal liver cells.23,25 In accordance with this, the expression of the canonical Wnt target genes Axin2, cMyc, and Ccnd1 was not induced in ETP/OP9-DW4 cocultures, showing that Wnt4 did not activate the canonical Wnt signaling pathway in ETPs or their immediate progeny. Furthermore, the absence of the transcription factor TCF1 (Tcf7), whose deletion has been shown to result in loss of canonical Wnt signaling in DN4 thymocytes and mature T lymphocytes,38,39 had no impact on the accumulation of ETPs and DN2s in the OP9-DW4 cocultures (Figure 3D). Although TCF1 is crucial for early T-cell development,26,40 compelling evidence suggests that TCF1 is not acting as an effector of canonical Wnt signaling in early T-cell development.38,40
Wnt4 regulates TEC numbers during fetal thymopoiesis and postnatal thymic expansion
Although our results indicate that Wnt4 has a direct effect on ETP and DN2 numbers, such an early defect would not be sufficient to explain a 2-fold difference in total cellularity because it should be compensated for at later stages.41 Given that the early stages of T-cell development are highly dependent on the availability of stromal niches,42 and because the number of TECs has been suggested to be the limiting factor in determining thymic size,43 we hypothesized that Wnt4 also could have an autocrine effect on TEC development.
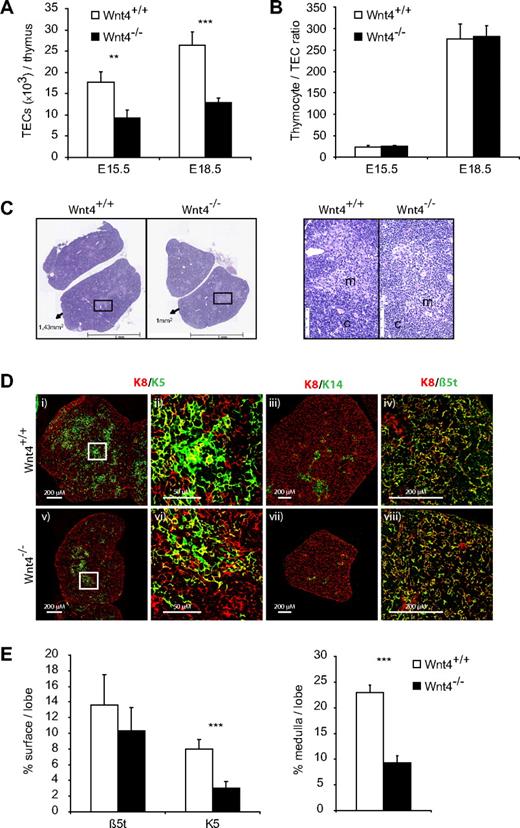
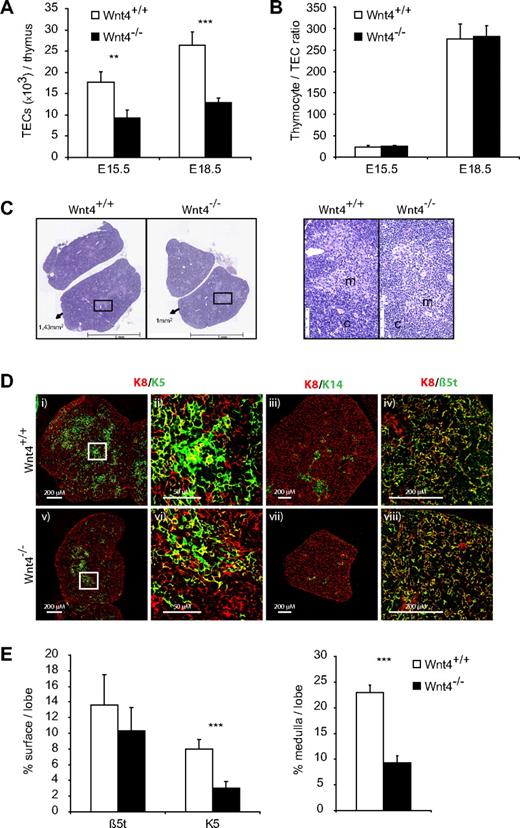
Indeed, there were fewer TECs per thymus already on E15.5 in the Wnt4−/− thymus and the relative difference remained the same on E18.5 (Figure 4A). The fold decrease in TEC numbers closely correlated with that of thymocytes, so that the thymocyte/TEC ratio was not altered between Wnt4+/+ and Wnt4−/− thymi (Figure 4B), suggesting that the difference in thymus size could be due to problems with TEC differentiation or maintenance. Histologic analysis of the E18.5 thymi revealed smaller medullary regions in the absence of Wnt4 (Figure 4C,E). The corticomedullary junction seemed less well defined in the Wnt4−/− thymus, whereas the nascent medulla contained more lymphocytes and fewer TECs (Figure 4C). These data indicate that the lack of Wnt4 during thymic organogenesis results in defective thymic architecture and delayed medullary development, the relevance of which is discussed further below.
Wnt4 controls fetal thymic size through regulation of TEC numbers. (A) TEC numbers in E15.5 and E18.5 Wnt4+/+ and Wnt4−/− thymi dissociated by enzymatic digestions and analyzed by flow cytometry. (B) Thymocyte to TEC ratio in E15.5 and E18.5 thymi. (C) Hematoxylin- and eosin-stained paraffin sections of thymi from Wnt4+/+ and Wnt4−/− mice at E18.5. Original magnification ×40. Higher magnification views of selected regions (black boxes) are shown on the right. m indicates medulla; c, cortex. (D) Frozen thymic sections from Wnt4+/+ and Wnt4−/− mice stained with anti-K8 (i-viii), anti-K5 (i,v), anti-K 14 (iii,vii), and anti-β5t (iv,viii). Higher magnification views of selected regions (white boxes) are shown for K8/K5-stained thymic sections (ii,vi). 10×/0.3 NA (i,iii,v,vii), 40×/1.3 NA oil objective (iv,viii), and 63×/1.4 NA oil objective (ii,vi). (E) Quantitative analysis of β5t and K5 staining (left panel) and the area occupied by medullary regions (right panel) per lobe of E18.5 Wnt4+/+ and Wnt4−/− thymi. Histograms represent mean ± SEM of 8-10 mice (*P < .05, **P < .01, ***P < .005).
Wnt4 controls fetal thymic size through regulation of TEC numbers. (A) TEC numbers in E15.5 and E18.5 Wnt4+/+ and Wnt4−/− thymi dissociated by enzymatic digestions and analyzed by flow cytometry. (B) Thymocyte to TEC ratio in E15.5 and E18.5 thymi. (C) Hematoxylin- and eosin-stained paraffin sections of thymi from Wnt4+/+ and Wnt4−/− mice at E18.5. Original magnification ×40. Higher magnification views of selected regions (black boxes) are shown on the right. m indicates medulla; c, cortex. (D) Frozen thymic sections from Wnt4+/+ and Wnt4−/− mice stained with anti-K8 (i-viii), anti-K5 (i,v), anti-K 14 (iii,vii), and anti-β5t (iv,viii). Higher magnification views of selected regions (white boxes) are shown for K8/K5-stained thymic sections (ii,vi). 10×/0.3 NA (i,iii,v,vii), 40×/1.3 NA oil objective (iv,viii), and 63×/1.4 NA oil objective (ii,vi). (E) Quantitative analysis of β5t and K5 staining (left panel) and the area occupied by medullary regions (right panel) per lobe of E18.5 Wnt4+/+ and Wnt4−/− thymi. Histograms represent mean ± SEM of 8-10 mice (*P < .05, **P < .01, ***P < .005).
To better quantify the medullary defect, we stained sections of E18.5 thymi for K8, K5, K14, and the thymus-specific proteasome unit β5t, the latter of which is exclusively expressed in mature cTECs.44 There was no difference in the density of K8 or β5t staining between Wnt4+/+ and Wnt4−/− thymi (Figure 4D-E); in contrast, the amount of K5 staining was decreased by ∼ 50%, and there was little detectable K14 in the absence of Wnt4. When we further analyzed the size of the K5+ medullary regions, they occupied a 2.5-fold smaller proportion of the total thymus (Figure 4E) in Wnt4−/− versus Wnt4+/+ thymi. These results confirmed the medullary TEC defect suggested by H&E staining. Together, our data indicate that Wnt4 influences both the quantity and organization of TECs during thymic organogenesis, thereby affecting thymic size and the earliest steps of thymocyte development.
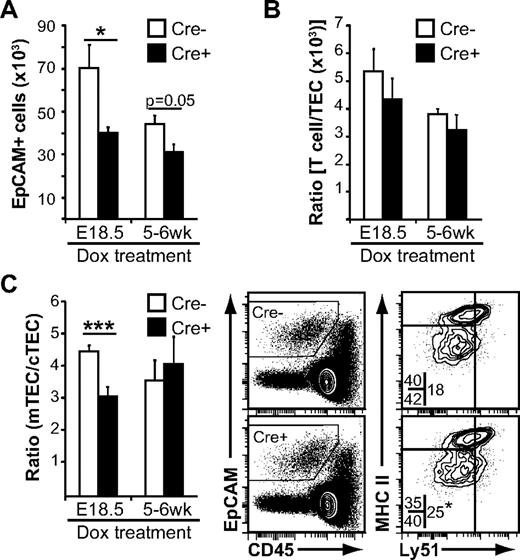
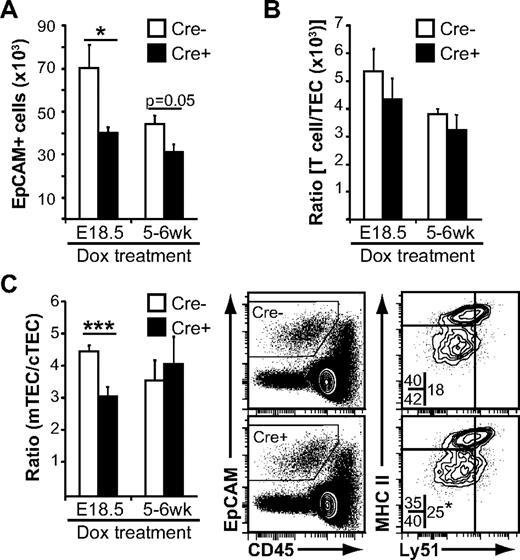
Given that the thymus undergoes massive expansion during the first month after birth, it seemed reasonable that Wnt4 could influence TEC expansion even in the early postnatal period. Indeed, the number of EpCAM+ cells detected by flow cytometry was lower in tetO-Cre ROSArtTA/+Wnt4lox/lox mice treated at birth compared with Cre− controls (Figure 5A). In accordance with what was observed in fetal thymi, the thymocyte/TEC ratio was unchanged (Figure 5B), suggesting that the decrease in thymic cellularity was once more mainly because of a defect in TEC expansion. Interestingly, there was a specific decrease in Ly51− mTECs (Figure 5C), resulting in a decreased mTEC/cTEC ratio, similar to what we observed in E18.5 fetal thymi (Figure 4D-E). When Wnt4 was deleted in the adult, the number of TECs recovered was once more smaller (Figure 5A), with no alteration to the thymocyte/TEC ratio (Figure 5B). In contrast to the fetal or early postnatal thymus, the mTEC/cTEC ratio was stable (Figure 5C). These results indicate that Wnt4 regulates TEC numbers in the fetal and postnatal thymus, and it has a specific role in mTECs during thymic expansion.
Loss of Wnt4 suppresses post-natal TEC expansion. Flow cytometry analysis of thymi from doxycycline-treated tetO-Cre:ROSArtTA/+:Wnt4fl/fl (Cre+) Wnt4-deficient mice and ROSArtTA/+:Wnt4fl/fl (Cre−) control littermates. (A) Numbers of EpCAM+ cells per thymus. (B) Thymocyte/TEC ratio from the same mice. (C) Analysis of the cortical (CD205+Ly51+) and the medullary (CD205−Ly51−) TEC subsets. The graph on the left shows the mTEC/cTEC ratio from Cre+ and Cre− mice. Representative FACS data for MHCII and Ly51 staining are shown on the right. The numbers within the FACS plots represent mean percentages of the different epithelial subsets over total EpCAM+ cells. The asterisk (bottom right plot) denotes statistical significance for the proportion of cTECs. All histograms represent mean ± SEM from 6-7 mice from at least 4 independent experiments (*P < .05, **P < .01, ***P < .005).
Loss of Wnt4 suppresses post-natal TEC expansion. Flow cytometry analysis of thymi from doxycycline-treated tetO-Cre:ROSArtTA/+:Wnt4fl/fl (Cre+) Wnt4-deficient mice and ROSArtTA/+:Wnt4fl/fl (Cre−) control littermates. (A) Numbers of EpCAM+ cells per thymus. (B) Thymocyte/TEC ratio from the same mice. (C) Analysis of the cortical (CD205+Ly51+) and the medullary (CD205−Ly51−) TEC subsets. The graph on the left shows the mTEC/cTEC ratio from Cre+ and Cre− mice. Representative FACS data for MHCII and Ly51 staining are shown on the right. The numbers within the FACS plots represent mean percentages of the different epithelial subsets over total EpCAM+ cells. The asterisk (bottom right plot) denotes statistical significance for the proportion of cTECs. All histograms represent mean ± SEM from 6-7 mice from at least 4 independent experiments (*P < .05, **P < .01, ***P < .005).
Decreased expression of Wnt4 has been linked to thymic senescence,45 and our own results show that exogenous Wnt4 facilitates thymic recovery post-HCT.23 We therefore compared thymi from E18.5, 1-month-old, 3-month-old, and 12-month-old Wnt4+/+ and Wnt4+/− mice to see whether decreased Wnt4 levels would accelerate thymic atrophy. Wnt4 haploinsufficiency had a significant effect on postnatal thymic expansion and up to 12 months of age, the cellularity of Wnt4+/− thymi remained inferior to that of Wnt4+/+ thymi (supplemental Figure 3). However, the pace of age-related thymic involution was similar in Wnt4+/+ and Wnt4+/− mice (supplemental Figure 3), further suggesting that the impact of Wnt4 was greater during thymic expansion than during its maintenance.
Lack of Wnt4 in the thymic stroma cannot be compensated for by Wnt4 sufficiency in the bone marrow microenvironment
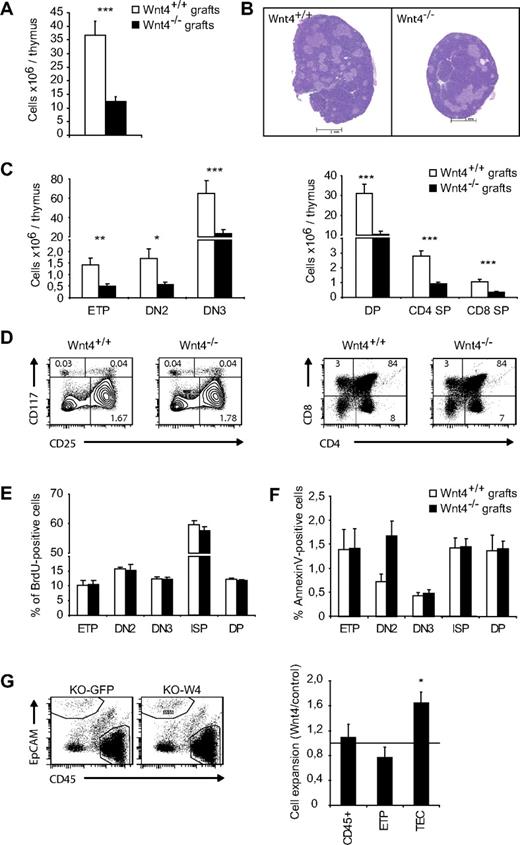
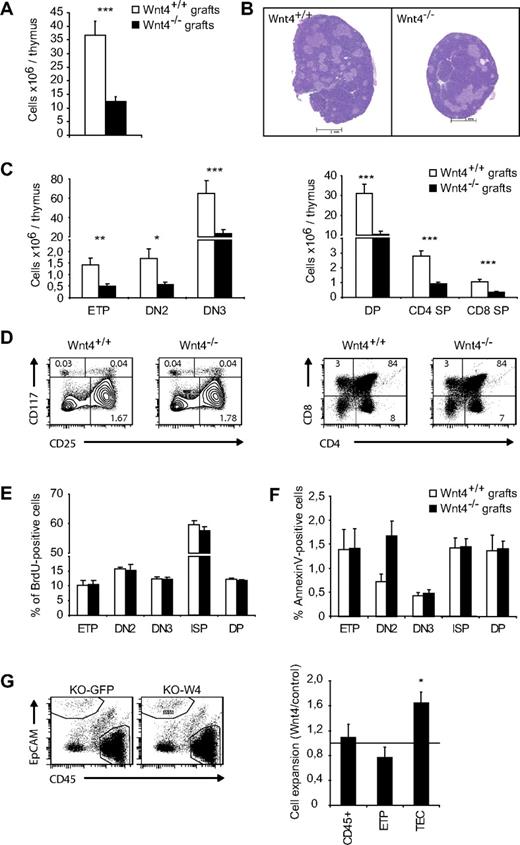
Wnt4 has been shown to play a positive role in the number of T-cell precursors in bone marrow and fetal liver.23,25 To evaluate the impact of intrathymic Wnt4 on thymopoiesis, we compared the development of wild-type progenitors in Wnt4+/+ and Wnt4−/− thymi. To this end, we grafted E18.5 thymic lobes under the kidney capsule of C57BL/6 hosts. Wnt4−/− lobes were visibly smaller and hypocellular after 4 weeks (Figure 6A-B), with no significant changes in thymocyte differentiation (Figure 6C-D), proliferation (Figure 6E), or apoptosis (Figure 6F). The expression of Wnt4 in the thymic stroma was therefore necessary for normal thymocyte numbers.
Wnt4-sufficiency in hematopoietic cells and extrathymic stromal cells does not rescue its absence in the thymic stroma. (A) Total thymic cellularity in Wnt4+/+ and Wnt4−/− grafts. (B) H&E-stained paraffin sections of Wnt4+/+ and Wnt4−/− thymic grafts. Original magnification ×40. (C) Numbers of thymocytes in Wnt4+/+ and Wnt4−/− grafts are depicted. (D) Representative flow cytometry data for cKit (CD117) and CD25 (gated on Linlo/neg cells for ETP, DN2, and DN3) and CD4/CD8 cell staining. The numbers within cytometry plots represent mean percentages of total. (E) Mice were injected intraperitoneally with 1 mg BrdU in PBS to provide a labeling pulse. Four hours later, thymic grafts were harvested, and BrdU uptake was detected with a BrdU flow kit (BD Biosciences Pharmingen). (F) Annexin V staining was performed to detect apoptotic T cells by flow cytometric analysis. (G) Representative flow cytometry data for Wnt4-overexpressing and control thymic aggregates (left panel). Graphs show the fold expansion of total CD45+ cells, CD25−cKithi (ETPs), and TECs in Wnt4 aggregates compared with controls (right panel). Histograms represent the mean ± SEM of 5-10 mice per group (*P < .05, **P < .01, ***P < .005).
Wnt4-sufficiency in hematopoietic cells and extrathymic stromal cells does not rescue its absence in the thymic stroma. (A) Total thymic cellularity in Wnt4+/+ and Wnt4−/− grafts. (B) H&E-stained paraffin sections of Wnt4+/+ and Wnt4−/− thymic grafts. Original magnification ×40. (C) Numbers of thymocytes in Wnt4+/+ and Wnt4−/− grafts are depicted. (D) Representative flow cytometry data for cKit (CD117) and CD25 (gated on Linlo/neg cells for ETP, DN2, and DN3) and CD4/CD8 cell staining. The numbers within cytometry plots represent mean percentages of total. (E) Mice were injected intraperitoneally with 1 mg BrdU in PBS to provide a labeling pulse. Four hours later, thymic grafts were harvested, and BrdU uptake was detected with a BrdU flow kit (BD Biosciences Pharmingen). (F) Annexin V staining was performed to detect apoptotic T cells by flow cytometric analysis. (G) Representative flow cytometry data for Wnt4-overexpressing and control thymic aggregates (left panel). Graphs show the fold expansion of total CD45+ cells, CD25−cKithi (ETPs), and TECs in Wnt4 aggregates compared with controls (right panel). Histograms represent the mean ± SEM of 5-10 mice per group (*P < .05, **P < .01, ***P < .005).
Regulation of TEC numbers by Wnt4
Finally, we wanted to understand the mechanism by which Wnt4 regulates TEC numbers. Wnt4 is the most strongly expressed Wnt in the MTS24+ immature TECs,22 suggesting that the differences could be more important in more immature cells (MTS24+). MTS24+ cells represent close to half of total TECs on E15.5.46 However, we saw no major differences in the expression of a panel of TEC-associated genes (supplemental Figure 4), nor did we detect any differences in proliferation or apoptosis between Wnt4+/+ and Wnt4−/− E15.5 TECs (supplemental Figure 4). The early differentiation also was normal as shown by comparable frequencies of CD40+CD205+ cTECs and CD40+CD205− mTECs between the 2 genotypes (supplemental Figure 4).
It remains formally possible that Wnt4 affects the proliferation, apoptosis, or both of a discrete subpopulation of TECs11 that our analysis on total EpCAM+ population does not reveal. Another possibility would be that Wnt4 regulates the TEC pool size by determining the type of cell divisions made by TEC stem cells: asymmetric division (maintenance), symmetric self-renewing division (expansion), or differentiating division (extinction).47 Addressing this question will require discovery of TEC stem cell specific markers. Nevertheless, when we wanted to evaluate whether exogenous Wnt4 was sufficient to increase TEC numbers, the production of Wnt4 by fibroblasts resulted in increased TEC recovery from 5-day thymic reaggregate cultures with no impact on ETPs or total thymocytes (Figure 6G), suggesting that the TEC expansion was a direct thymocyte-independent effect of Wnt4.
Discussion
Using both conventional and conditional null mutant mice, we demonstrate here that Wnt4 regulates steady-state thymic cellularity by a TEC-dependent mechanism. The absence of Wnt4 suppressed fetal and early postnatal thymic expansion, resulted in decreased TEC numbers, an alteration of the medullary-to-cortical TEC ratio, and a disproportionate loss of the most immature cKithi thymic precursors. Wnt4 also is implicated in the maintenance of adult thymopoiesis, although the impact of its deletion once the thymic decline has started is more subtle. Together, our results show that Wnt4 controls thymic size by modulating TEC expansion and the early TEC-dependent thymocyte expansion, both in the fetal and postnatal thymus.
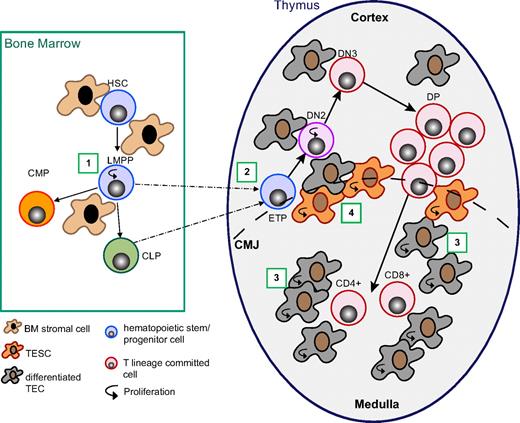
The regulation of thymic cellularity is dependent in part on thymocyte-intrinsic factors,42 and in part on the capacity of the thymic stroma to support the earliest steps of thymopoiesis.42,43,48 Our results clearly indicate that Wnt4 regulates the number of TECs in the thymus and consequently the number of intrathymic niches available for thymus-seeding progenitors (Figures 4A, 5A, and 6). Conversely, Wnt4 also is capable of directly influencing the expansion of the most immature cKithi thymocytes in vitro (Figure 3C-D). Given that the lack of Wnt4 did not affect the expression of growth factors and cytokines important for early thymopoiesis (supplemental Figure 4), we hypothesize that the disproportionate decrease in ETPs and DN2s in Wnt4-deficient thymi (Figure 2C-E) was in large part directly because of the lack of Wnt4 signals from the surrounding stroma. We have identified a prethymic role for Wnt4 as well; lack of Wnt4 diminishes progenitor cell expansion in the fetal liver and bone marrow with the most pronounced effect on lymphoid-primed multipotential progenitors23,25 that display the highest T-cell progenitor frequency. Our results presented here show that the lack of Wnt4 in the thymic stroma is sufficient for suboptimal thymic cellularity; however, the prethymic effect may be significant on its own under certain circumstances, such as post-HCT when progenitor cells are undergoing massive expansion. Thus, Wnt4 acts on both prethymic and intrathymic T-cell precursors as well as on the thymic stromal environment with the net effect of promoting thymopoiesis. An illustration of the multiple pre- and intrathymic effects of Wnt4 is presented in Figure 7.
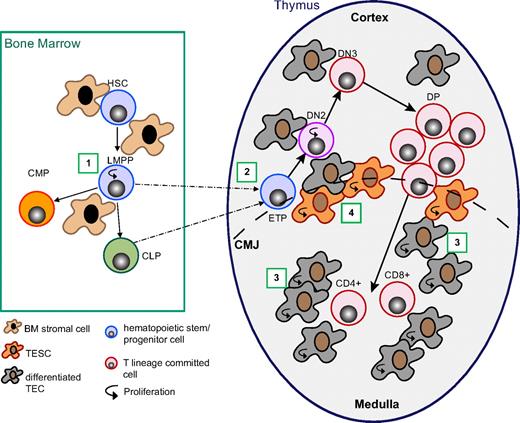
Wnt4 regulates thymopoiesis at multiple stages. Working model depicting the different levels of Wnt4 action on T-cell development. (1) Wnt4 in the BM increases lymphoid-primed multipotential progenitor (LMPP) survival in a paracrine manner.23,25 (2) Wnt4 facilitates ETP/DN2 expansion in a paracrine manner (Figures 2–3). (3) Wnt4 facilitates TEC expansion, in particular that of mTECs in the fetal and early postnatal period, probably in an autocrine manner (Figures 4–5). (4) Wnt4 is likely to increase the clonal expansion capacity of precursor TECs (Figures 4,6). HSC indicates hematopoietic stem cell; CLP, common lymphoid precursor; CMP, common myeloid precursor; TESC, thymic epithelial stem/progenitor cell; CMJ, corticomedullary junction.
Wnt4 regulates thymopoiesis at multiple stages. Working model depicting the different levels of Wnt4 action on T-cell development. (1) Wnt4 in the BM increases lymphoid-primed multipotential progenitor (LMPP) survival in a paracrine manner.23,25 (2) Wnt4 facilitates ETP/DN2 expansion in a paracrine manner (Figures 2–3). (3) Wnt4 facilitates TEC expansion, in particular that of mTECs in the fetal and early postnatal period, probably in an autocrine manner (Figures 4–5). (4) Wnt4 is likely to increase the clonal expansion capacity of precursor TECs (Figures 4,6). HSC indicates hematopoietic stem cell; CLP, common lymphoid precursor; CMP, common myeloid precursor; TESC, thymic epithelial stem/progenitor cell; CMJ, corticomedullary junction.
The numbers of mTECs and cTECs are differentially regulated by Wnt4 (Figures 4D and 5C). One major regulator of TEC differentiation is the availability of cross talk from the developing thymocytes. Even if the initial appearance of cTECs is thymocyte independent, their differentiation and maturation seem dependent on the presence of DN thymocytes.11,16,49 Although the lack of Wnt4 results in decreased DN thymocyte numbers, they would seem sufficient to drive cTEC maturation, because we detected no significant changes in the proportion of CD205+CD40+ cTECs in the E15.5 thymus (supplemental Figure 4) or K8/β5t staining on E18.5 (Figure 4D). In comparison, K5+ and K14+ mTEC staining was reduced in the E18.5 Wnt4−/− thymus (Figure 4D), and the number of CD205−Ly51− TECs was reduced by ∼ 50% in the early postnatal thymus in the absence of Wnt4 (Figure 5). mTEC development is dependent on signals from positively selected thymocytes (in the postnatal medulla) and CD4+ CD3− lymphoid tissue inducer cells (during embryogenesis). Although there is a net decrease in the number of CD4+ and CD8+ thymocytes in the Wnt4-deficient thymus (supplemental Figure 2), their proportion is not significantly reduced and thus the relative number of single positive thymocytes with respect to the volume of the thymic medulla is unlikely to be changed. Moreover, there was no delay in the appearance of DP or single-positive thymocytes in the Wnt4−/− fetal thymus (data not shown).
We have shown previously in hematopoietic cells that although Wnt4 does not seem to have a proproliferative effect in itself, its impact is strongest on populations that are proliferating.23,25 In that respect, it follows that the loss of Wnt4 resulted in a more dramatic phenotype in fetal and early postnatal thymus, which are still in the expansion phase, than in the adult thymus. It is also interesting that the thymic grafts showed the most important fold change in total cellularity: there is massive apoptosis and remodeling in the graft,50 which requires expansion by the remaining TECs to reestablish the thymic microenvironment. Wnt4 could thus impact the capacity for clonal expansion of epithelial progenitor cells. mTECs also have been shown to proliferate more than cTECs31 ; hence, the lack of Wnt4 might be seen more strongly in mTECs. Consistent with the latter hypothesis, decrease in the mTEC/cTEC ratio is observed in normal age-related thymic involution in wild-type mice and in pathologic thymic atrophy in several mutant mice.13,14,31
In conclusion, we show here that Wnt4 regulates the expansion of both immature thymocytes and TECs with the net effect of increasing total cellularity in the fetal as well as early postnatal and adult thymus. Together with our previous report on the role of Wnt4 in thymic recovery post-HCT,23 our data suggest that Wnt4, or its downstream effectors, would make very interesting candidates for countering thymic insufficiency and atrophy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Danièle Gagné and Gaël Dulude for advice on flow cytometry and cell sorting, to Pierre Chagnon and IRIC's Genomics Core Facility for valuable advice and help with qPCR, and to the staff of IRIC animal care facility for superb assistance.
This study was funded by the Canadian Institutes of Health Research (CIHR; grant MOP42384). K.M.H. was supported by a training grant from the Terry Fox Foundation. C.P. holds a Canada Research Chair in Immunobiology.
Authorship
Contribution: K.M.H., J.R.V., and S.B. designed and conducted experiments and analyzed data; J.S. and S.V. contributed vital reagents; and K.M.H., J.R.V., and C.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claude Perreault, Institute for Research in Immunology and Cancer, PO Box 6128, Station Centre-Ville, Montreal, QC, Canada, H3C 3J7; e-mail: c.perreault@videotron.ca.
References
Author notes
K.M.H. and J.R.V. contributed equally to this study.