Abstract
Human mesenchymal stem/progenitor cells (hMSCs) repair tissues and modulate immune systems but the mechanisms are not fully understood. We demonstrated that hMSCs are activated by inflammatory signals to secrete the anti-inflammatory protein, TNF-α–stimulated gene 6 protein (TSG-6) and thereby create a negative feedback loop that reduces inflammation in zymosan-induced peritonitis. The results demonstrate for the first time that TSG-6 interacts through the CD44 receptor on resident macrophages to decrease zymosan/TLR2-mediated nuclear translocation of the NF-κB. The negative feedback loop created by MSCs through TSG-6 attenuates the inflammatory cascade that is initiated by resident macrophages and then amplified by mesothelial cells and probably other cells of the peritoneum. Because inflammation underlies many pathologic processes, including immune responses, the results may explain the beneficial effects of MSCs and TSG-6 in several disease models.
Introduction
Considerable efforts are currently being made to explore the therapeutic potentials of the stem/progenitor cells from bone marrow referred to initially as colony forming units-fibroblastic, then as marrow stromal cells, subsequently as mesenchymal stem cells, and most recently as multipotent mesenchymal stromal cells (MSCs).1-6 The cells are readily isolated from small aspirates of bone marrow from normal human donors or patients, they expand rapidly for 30 or more population doublings in culture, and they can differentiate into several cellular phenotypes in culture and in vivo. The therapeutic potentials of the cells have been tested in animal models and in clinical trials for a large number of diseases (see www.clinicaltrials.gov). Initially, it was assumed that the cells repaired tissues by engrafting and differentiating to replace injured cells. Engraftment with differentiation was observed in some animal models such as those with severe injuries to tissues, in embryos, or with local infusions of high concentrations of the cells. In most experimental situations, however, repair with functional improvements was observed without evidence of long-term engraftment. Therefore, most of the beneficial effects were explained by paracrine secretions or cell-to-cell contacts that had multiple effects including modulation of inflammatory or immune reactions.6-10 Of special importance were the observations that although MSCs in culture secreted a large number of cytokines,11,12 they were activated by cross-talk with injured cells to express high levels of additional therapeutic factors.10,13
Previously we observed14 that intravenously infused human MSCs (hMSCs) improved a mouse model for myocardial infarction in part because the hMSCs were trapped in the lung as microemboli and the injury produced by the microemboli activated the cells to secrete the anti-inflammatory protein TNF-α–stimulated gene 6 protein (TSG-6). TSG-6 suppressed inflammatory reactions triggered by ischemia in the heart and thereby limited destruction of cardiomyocytes by invading neutrophils and monocytes/macrophages.
TSG-6 is a 30 kD glycoprotein that was shown to produce anti-inflammatory effects in several animal models.15,16 In transgenic mice, inactivation of the gene increased inflammatory responses,17 and over-expression of the gene decreased inflammatory responses.18 In addition, administration of the recombinant protein improved arthritis and decreased inflammation in several murine models.19,20
To better understand the anti-inflammatory effects of hMSCs, we used the model of zymosan-induced peritonitis in mice.21,22 The results indicated that hMSCs decreased inflammation in the model in part because the hMSCs were activated by the initial inflammatory microenvironment of the peritoneal cavity to secrete TSG-6. TSG-6 then produced a CD44-dependent decrease in the zymosan/TLR2-mediated stimulation of NF-κB signaling in resident macrophages. In effect, the hMSCs and TSG-6 generated a negative-feedback loop that attenuated the cascade whereby the pro-inflammatory signals from resident macrophages were amplified by mesothelial cells and probably other cells of the peritoneum to increase neutrophil recruitment.23
Methods
hMSC preparation
Frozen vials of hMSCs from bone marrow were obtained from the Center for the Preparation and Distribution of Adult Stem Cells (formerly http://www.som.tulane.edu/gene_therapy/distribute.shtml; currently http://medicine.tamhsc.edu/irm/msc-distribution.html) that supplies standardized preparations of MSCs enriched for early progenitor cells to > 300 laboratories under the auspices of a National Institutes of Health (NIH)/National Center for Research Resources grant (P40 RR 17 447-06). Most of the experiments were performed with hMSCs from donor 5068 but some were repeated with hMSCs from 3 other donors (7075, 7027, and 7009). To expand hMSCs,24 a frozen vial of 1.0 × 106 passage 2 cells was thawed, and plated at 200 cells/cm2 in multiple 150 mm plates with 30 mL complete culture medium (CCM) that consisted of α-minimal essential medium (α-MEM; Invitrogen), 17% FBS (lot-selected for rapid growth of MSCs; Atlanta Biologicals), 100 units/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen), and 2 mM l-glutamine (Invitrogen). In transfection experiments, the antibiotics were omitted from the CCM. The cultures were incubated, and the medium replaced every 2 days for approximately 5 days until they were 70% confluent. The medium was discarded, the cultures were washed with PBS, adherent cells were harvested with 0.25% trypsin and 1mM ethylenediaminetetraacetic acid for 3 minutes at 37°C, and the cells were resuspended at 1.6 × 106 cells in 160 μL of sterile HBSS for injection.
Cell lines and culture conditions
Source and conditions for culture of murine macrophage-like cell line (RAW 264.7), human mesothelial cells (MeT-5A, ATCC) and human embryonic kidney 293 cells are presented in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Animals
Six- to 8-week-old male wild-type (C57BL/6J) and male transgenic mice with a CD44 knockout (Stock #005085: B6.Cg-Cd44tm1Hbg/J) were purchased from The Jackson Laboratory and used under a protocol approved by the Institutional Animal Care and Use Committee of Texas A&M Health Science Center, College of Medicine. Mice were kept on a 12-hour light/dark cycle and fed and watered ad libitum.
Zymosan-induced peritoneal inflammation
Mice were anesthetized with 4% isofluorane in 100% oxygen and were injected intraperitoneally (IP) with 1 mg of zymosan A (Sigma-Aldrich) in 1 mL of PBS.21,22 Before use, zymosan was suspended in PBS at 1 mg/mL and autoclaved at 121°C for 15 minutes. After cooling the sample to room temperature for 1 hour, zymosan was recovered by centrifugation at 300g for 10 minutes at 4°C, washed 3 times by recentrifugation with ice cold sterile PBS, and then resuspended in PBS at 1 mg/mL for injection. Fifteen minutes after zymosan administration, the mice were infused IP with either 1.6 × 106 hMSCs in 160 μL of HBSS, 30 μg rhTSG-6 (catalog #2104-TS; R&D Systems) in 160 μL of PBS, or vehicle controls of HBSS or PBS. At times indicated (2, 4, 8, 24, 48, and 72 hours after zymosan injection), mice were euthanized by lethal dose of 5% isofluorane in 100% oxygen followed by cervical dislocation. Peritoneal exudates were collected by lavage twice with 5 mL of sterile PBS. The cells were recovered by centrifugation at 300 g for 10 minutes at 4°C followed by incubation in 5 mL of RBC lysis buffer (eBioscience) for 5 minutes at room temperature. The samples were filtered through a cell strainer (70 μm mesh) and the cells were recovered by centrifugation at 300 g for 10 minutes at 4°C. For assays of polymorphonuclear cells (PMN) and mononuclear cells, 0.5 × 106 cells were blocked for 5 minutes at room temperature in 0.5 mL of PBS containing 0.5 μg of anti–mouse CD16/32 (Clone 93; eBiosciences) and washed twice by centrifugation with PBS. Labeled cells were incubated in 0.5 mL PBS with 0.5 μg FITC-conjugated anti–mouse GR-1 (Ly-6G; Clone 1A8; BD Pharmingen) and 0.5 μg phycoerythrin-Cy7-conjugated anti–mouse F4/80 (Clone BM8; eBiosciences) for 20 minutes at room temperature, and then washed with 5 mL of PBS followed by centrifugation before assay by flow cytometry (Cytomic FC500). For assays of peritoneal cytokines, the first 5 mL peritoneal lavage fluid was clarified by centrifugation at 500 g for 5 minutes followed by filtration (0.22 μm PVDF syringe filter; Millipore). The samples were concentrated 10-fold by ultrafiltration (Ultracell-5K; Millipore) and TNF-α was assayed using a mouse-specific TNF-α ELISA (R&D Systems). For assays of HEK-hTLR2 cells stably transfected to express CD44, 0.5 × 106 of HEK-hTLR2-CD44 or HEK-hTLR2-pcDNA cells were stained with Ag-presenting cell–conjugated anti–human CD44 (Clone G44-26, BD Pharmingen) and assayed by flow cytometry.
Activation of hMSCs with TNF-α to secrete TSG-6
Passage 2 hMSCs were plated at 5000 cells/cm2 in 150 mm plates with 30 mL CCM and incubated for 1 day. The medium was then changed to α-MEM containing 2% heat inactivated FBS and 10 ng/mL hTNF-α (R&D Systems). The cultures were incubated for 18 hours, and then lifted with 0.25% trypsin with 1mM EDTA for 3 minutes at 37°C. To confirm increased expression of TSG-6, RNA was extracted from aliquots of the cells (RNeasy Mini Kit; QIAGEN) and assayed for TSG-6 expression by real-time RT-PCR as described previously.14
Transfection of hMSCs with TSG-6 siRNA
A frozen vial of 1.0 × 106 passage 2 hMSCs was thawed, and plated at 200 cells/cm2 in multiple 150 mm plates with 30 mL CCM lacking antibiotics. The cultures were incubated with replacement of medium every 2 days. After incubation for 4 days, the cells were transfected with siRNA for TSG-6 (sc-39 819; Santa Cruz Biotechnology) or negative control (Stealth RNAi Negative Control; Invitrogen) with a commercial kit (Lipofectamine RNAiMAX reagent; Invitrogen). A mixture was prepared of (1) 63.6 μL of a 10 μM stock solution of siRNA for TSG-6 (sc-39 819; Santa Cruz Biotechnology) or negative control (Stealth RNAi Negative Control; Invitrogen) diluted with 2.65 mL of transfection medium (OptiMEM I Medium; Invitrogen), and (2) 79.5 μL of transfection reagent (Lipofectamine RNAiMAX Reagent; Invitrogen) diluted with 2.65 mL of transfection medium (OptiMEM I Medium). The mixture was incubated for 20 minutes at room temperature. The mixture (5.42 mL) together with 26.5 mL of transfection medium (OptiMEM I Medium) was added to the cells. Six hours later, the medium was replaced with 30 mL CCM lacking antibiotics and hMSCs were incubated for 16 to 20 hours. The cells were harvested with 0.25% trypsin and 1 mM EDTA for 3 minutes at 37°C, and resuspended at 1.6 × 106 cells in 160 μL sterile HBSS for injection. To confirm knockdown of TSG-6, RNA was extracted from aliquots of the cells (RNeasy Mini Kit; QIAGEN) and assayed for TSG-6 by real-time RT-PCR.14
Cocultures of macrophages with hMSCs and TSG-6
For the cocultures, murine macrophages (RAW264.7) were plated at approximately 1.0 × 105 cells/cm2 in 6-well microplates (Corning) and incubated for 4 hours in 2 mL α-MEM containing 2% heat-inactivated FBS and 20 μg/mL zymosan and with one of the following: (1) 1.0 × 105 hMSCs; (2) 1.0 × 105 hMSCs activated with TNF-α; (3) 1.0 × 105 activated hMSCs transduced with scrambled siRNA; (4) 1.0 × 105 activated hMSCs transfected with TSG-6 siRNA, or (5) control media. To improve reproducibility, a stock solution of zymosan was prepared by heating 20 mg/mL of zymosan in HBSS at 100°C for 20 minutes. After being cooled to room temperature, zymosan was disaggregated by sonication and washed twice by centrifugation with cold sterile HBSS. Zymosan was suspended at 20 mg/mL in HBSS and aliquots were stored at −80°C. The samples were thawed just before use.
Real-time RT-PCR and ELISA assays
Approximately 200 ng of total RNA from the cell cultures was used to synthesize double-stranded complementary DNA by reverse transcription (SuperScript III, Invitrogen). The complementary DNA was analyzed by real-time RT-PCR (ABI 7900 Sequence Detector, Applied Biosystems). For assays of mouse-specific transcripts, mouse-specific primers and probes (Applied Biosystems) were used: TNF-α (Mm00443258_m1), IL-6 (Mm99999064_m1), IL-10 (Mm99999062_m1), Cxcl1 (Mm01354329_g1) and Cxcl2 (Mm00436450_m1). For relative quantitation of gene expression, mouse-specific glyceraldehyde-3-phosphate dehydrogenase primer and probes (Mm99999915_g1) were used. For assays of human-specific transcripts, human-specific primers and probes were used: IL-6 (Hs00174131_m1), IL-8 (Hs01567912_g1), and CCL2 (Hs00234140_m1). For assays of HEK-hTLR2 cells stably transfected to express CD44, the primers and probe to detect CD44 expression were Hs01075861_m1. For the assays, reactions were incubated at 95°C for 20 seconds, and then 40 cycles at 95°C for 1 second followed by 60°C for 20 seconds using Taqman Fast Universal PCR Master Mix (Applied Biosystems).
For assay of IL-6 in mouse plasma, mice were killed at 8 hours after injection of zymosan and HBSS, 1.0 × 106 hMSCs or 30 μg rhTSG-6, blood was obtained by cardiac puncture, and plasma was assayed with a commercial kit (Mouse IL-6 Quantikine ELISA Kit; R&D Systems).
NF-κB translocation assays
Murine macrophages (RAW 264.7) were plated at 1.4 × 105 cells/cm2 in 8-well chamber slides (Lab-Tek II Chamber Slide; Nalge Nunc) and incubated for 1 hour in 0.2 mL of 2% heat inactivated FBS in α-MEM with or without 20 μg/mL zymosan and with and without 10 or 150 ng/mL rhTSG-6. The cells were washed twice with PBS followed by centrifugation and were fixed with 100% methanol for 5 minutes. The cells were washed with PBS followed by centrifugation and blocked with 5% BSA in PBS and incubated with 1 μg/mL of anti–NF-κB p65 antibody (ab16502, Abcam) in blocking buffer (Image-iT FX Signal Enhancer; Invitrogen) overnight at 4°C. The samples were then incubated for 1 hour with 1:1000 dilution of the stock solution of 2 mg/mL anti–rabbit IgG secondary antibody (Alexa Fluor 488 goat; Invitrogen). DAPI (VECTASHIELD Mounting Medium) was used to stain the cell nuclei. The slides were visualized with fluorescent microscopy (Eclipse 80i; Nikon). For each sample, 3 fields with at least 70 cells per field were captured at random and quantified (ImageJ Version 1.44 software; NIH, http://rsbweb.nih.gov/ij/).
Cocultures of macrophages with mesothelial cells
For coculture assays, human mesothelial cells (Met-5A cells, ATCC) were seeded at 7500 cells/cm2 in 12-well microplates (Corning) with 1 mL growth medium. Three days later, the medium was replaced with 1 mL α-MEM containing 2% heat-inactivated FBS and samples were cultured for 2, 4, and 8 hours with or without addition of 1 000 murine macrophages (RAW264.7), 20 μg/mL zymosan, or rhTSG-6 (10 or 100 ng/mL). RNA was isolated for assays (RNeasy Mini Kit; QIAGEN).
Establishment of stable NF-κB reporter cell expressing CD44
The CD44 coding sequence (Origene #SC128160) was inserted into the plasmid pcDNA 3.1 (Invitrogen) using Not1/Xba1 sites and the plasmid (pcDNA 3.1-CD44) transformed into Escherichia coli (Subcloning Efficiency DH5a Competent Cells; Invitrogen) for cloning. HEK-Blue-hTLR2 cells were plated at a density of 6.0 × 105 cells per 10 cm culture dish (Corning) in 10 mL of growth media lacking antibiotics. After incubation for 1 day, the cells were transfected with the expression plasmid (pcDNA 3.1-CD44) or pcDNA 3.1 with a commercial kit (Lipofectamine 2000; Invitrogen). A mixture of (1) 24 μg of pcDNA 3.1-CD44 or pcDNA control vector diluted with 1.5 mL of medium (OptiMEM I Medium), and (2) 60 μL of transfection reagent (Lipofectamine 2000) diluted with 1.5 mL of transfection medium (OptiMEM I Medium) was incubated for 20 minutes at room temperature. The mixture (3 mL) was added to the cells with 15 mL of transfection medium (OptiMEM I Medium). Six hours later, the medium was replaced with 10 mL per well of growth media lacking antibiotics and the cells were incubated for 16 to 20 hours. The transfected cells were lifted by pipetting and plated in four 10 cm culture dishes in 10 mL of growth medium supplemented with 1× HEK-Blue Selection media (InvivoGen). The cells were kept in the selection media for 2 weeks and individual clones designated as HEK-hTLR2-CD44 and HEK-hTLR2-pcDNA were selected for expansion. To confirm the expression of CD44 by immunocytochemistry, the cells (5.0 × 104 cells/cm2) were plated on 96-well poly-d-lysine precoated plates (BD Pharmingen), washed with PBS, and blocked with blocking solution (PBS supplemented with 5% horse serum) for 15 minutes at room temperature. After removing the blocking solution, the cells were incubated with an anti-CD44 antibody (100 ng/mL; sc-59757, Santa Cruz Biotechnology Inc) for 30 minutes at room temperature. After subsequent washing with PBS, the cells were stained with Alexa Fluor 488-labeled goat anti–mouse IgG antibody (2 μg/mL; Molecular Probes) in blocking solution for 30 minutes at room temperature. The cells were visualized with fluorescent microscopy (Eclipse Ti-S; Nikon). CD44 expression in HEK-hTLR2-CD44 cell line was also confirmed by real time RT-PCR and FACS analysis (supplemental Figure 3).
Assays with a NF-κB reporter cells expressing CD44
Cells were plated at 3.0 × 104 cells/cm2 in 96 well poly-D–lysine coated microplates (BD Pharmingen) in 100 μL of growth media lacking antibiotics and supplemented with 1× HEK-Blue Selection media (InvivoGen) to retain the transfected plasmids. Cells were stimulated by incubation for 7 hours with 20 μg/mL zymosan and with or without rhTSG-6 in 100 μL of HEK-Blue Detection media (InvivoGen) containing 2% heat-inactivated FBS. Absorbance was assayed at 650 nm with a plate reader. The supernatant was carefully removed, and the cells on the microplates were frozen and stored at −80°C. The cell number was determined by CyQUANT Cell Proliferation Assay Kit (Invitrogen).
Assays in NF-κB reporter cell line expressing CD44 and genes downstream of TLR2
To prepare reporter cells over-expressing downstream genes of TLR2, HEK-hTLR2-CD44 were transfected with pCMV-SPORT6 MyD88 (MHS1010-73 828; Open Biosystems), pCMV-SPORT-TIRAP (MHS1010-7508115; Open Biosystems), or pcDNA3.1 (Invitrogen) using Lipofectamine 2000 as described above for transfection of CD44. Briefly, HEK-Blue-hTLR2-CD44 cells were plated at 1.0 × 104 cells/cm2 in 10 cm culture dishes (Corning) in 10 mL of growth media lacking antibiotics. After incubation for 1 day, the cells were transfected with 10 μg of plasmid and 60 μL of lipofectamine reagent for 4 hours. One day after transfection, cells were plated at 3.0 × 104 cells/cm2 in 96 well poly-d-lysine coated microplates (BD Pharmingen) in 100 μL of growth media lacking antibiotics. The NF-κB reporter assay was performed as previously described.
Effects of a blocking antibody to CD44 in macrophages
RAW 264.7 were plated at 1.0 × 105 cells/cm2 in 6-well microplates (Corning) in 2 mL growth medium. The samples were incubated for 15 minutes with 2.5 μg/mL of an anti-CD44 antibody (Clone KM81; Lifespan Biosciences) or 2.5 μg/mL of rat isotope control (IgG2a, BD Pharmingen) and then incubated for 4 hours with 20 μg/mL zymosan with or without 100 ng/mL rhTSG-6 or 1.0 × 105 hMSCs activated with TNF-α.
Isolation of resident macrophage RNA
Peritoneal lavage fluid was harvested with 10 mL PBS 2 hours after zymosan injection. Cells were collected by centrifugation at 300g for 10 minutes and incubated in 0.1 mL of PBS containing 2 μg phycoerythrin-Cy7-conjugated anti–mouse F4/80 (Clone BM8; eBiosciences) and 10 μL anti–mouse CD11b microbeads (Miltenyibiotech) for 20 minutes at room temperature. Cells were washed with 1 mL PBS and centrifuged at 300g at 4°C for 5 minutes, resuspended in 1 mL of buffer (AutoMACS Running Buffer; Miltenyi Biotech), and CD11b positive cells were isolated using a magnetic column (MACS Separation Column; Miltenyi Biotech). The isolated cells were further sorted for F4/80 positive cells by FACS (Moflo XDP sorter; Beckman Coulter). Sorted cells were lysed immediately using 2 mL of lysis buffer and RNA was isolated (RNeasy Mini Kit; QIAGEN) for assays.
Statistical analyses
Comparisons between 2 groups were made with the use of unpaired and 2-tailed Student t tests. P < .05 was considered significant.
Results
hMSCs and TSGs-6 reduced zymosan-induced peritonitis
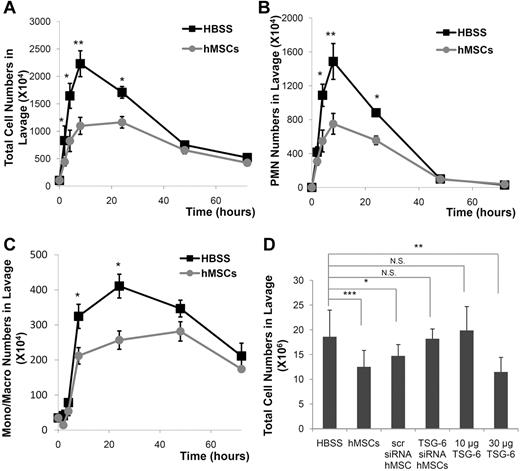
Intraperitoneal infusion of zymosan produced a prompt peritonitis in the mice as indicated by an increase in the lavage fluid of both total cells and PMNs beginning at 2 hours (Figure 1A-B). As expected,25 PMNs were the first effector cells recruited into the exudate followed by mononuclear cells. PMNs reached a peak level in approximately 8 hours and then decreased with a half-life of approximately 30 hours. In contrast, monocytes/macrophages increased more slowly, reached a peak level at 24 hours, and persisted at a high level for > 60 hours (Figure 1C). Intraperitoneal infusion of 1.6 × 106 hMSCs 15 minutes after infusion of the zymosan decreased the total number of cells, the number of PMNs, and the number of monocytes/ macrophages (Figure 1A-D). To test the hypothesis that the anti-inflammatory effects of hMSCs in zymosan-induced peritonitis were largely explained by action of TSG-6, we knocked down expression of the gene with TSG-6 siRNA (supplemental Figure 1B). The hMSCs transduced with the siRNA had no significant effect on the inflammatory response to zymosan (Figure 1D). In addition, the anti-inflammatory effects of hMSCs were largely reproduced by intraperitoneal infusion of 30 μg rhTSG-6 instead of hMSCs (Figure 1D).
Human MSCs and rhTSG-6 decreased the inflammatory response to zymosan-induced peritonitis. (A) Total cells in the lavage fluid. (B) Polymorphonuclear cells (PMN) in the lavage fluid. (C) Monocytes/macrophages in the lavage fluid. (D) Total cells in the lavage fluid harvested at 4 hours after administration of hMSCs, control hMSCs transfected with a scrambled siRNA (scr), hMSCs transfected with an siRNA for TSG-6, or rhTSG-6. Values are mean ± SD (n = 5 mice per time point; *P < .05; **P < .005; ***P < .0005).
Human MSCs and rhTSG-6 decreased the inflammatory response to zymosan-induced peritonitis. (A) Total cells in the lavage fluid. (B) Polymorphonuclear cells (PMN) in the lavage fluid. (C) Monocytes/macrophages in the lavage fluid. (D) Total cells in the lavage fluid harvested at 4 hours after administration of hMSCs, control hMSCs transfected with a scrambled siRNA (scr), hMSCs transfected with an siRNA for TSG-6, or rhTSG-6. Values are mean ± SD (n = 5 mice per time point; *P < .05; **P < .005; ***P < .0005).
hMSCs and TSG-6 decreased stimulation of macrophages
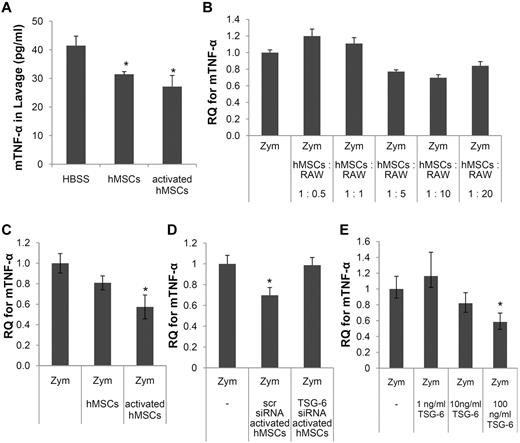
To explore the effects of hMSCs and rhTSG-6 on macrophages, coculture experiments were performed with a line of mouse macrophages, RAW264.7.26 The strategy enabled us to simulate the in vivo conditions (Figure 1) and to use species-specific probes to assay gene expression changes in the hMSCs and the murine macrophages. We first established that the macrophages were stimulated by zymosan to express mTNF-α in a dose-dependent manner (supplemental Figure 2). We then carried out a preliminary experiment to establish an appropriate ratio of the 2 cell types for the following experiments. Ratios of 1:5 and 1:10 were selected for future experiments. At both ratios, the hMSCs decreased the expression of mTNF-α by the macrophages (Figure 2B).
Inhibition of zymosan-induced TNF-α expression in the peritoneum and in cultured macrophages. (A) ELISAs for mTNF-α in lavage fluid. Values are mean ± SD (n = 3; *P < .05). (B) Mouse-specific real-time RT-PCR assays for mTNF-α in macrophages stimulated with zymosan with or without various numbers of hMSCs. Data are expressed as mean and range of 2 values. (C) As in panel B except that the cocultures contained hMSCs (1:10 ratio to macrophages) and the hMSCs were either standard preparations or hMSCs activated to express TSG-6 by incubation with hTNF-α. (D) As in panel C except that the hMSCs were either activated hMSCs transfected with scrambled siRNA (scr) or activated hMSCs transfected with an siRNA for TSG-6. (E) As in panel D except murine macrophages cultured with zymosan with or without TSG-6. Values in panels C, D, and E are mean ± SD (n = 3; *P < .05).
Inhibition of zymosan-induced TNF-α expression in the peritoneum and in cultured macrophages. (A) ELISAs for mTNF-α in lavage fluid. Values are mean ± SD (n = 3; *P < .05). (B) Mouse-specific real-time RT-PCR assays for mTNF-α in macrophages stimulated with zymosan with or without various numbers of hMSCs. Data are expressed as mean and range of 2 values. (C) As in panel B except that the cocultures contained hMSCs (1:10 ratio to macrophages) and the hMSCs were either standard preparations or hMSCs activated to express TSG-6 by incubation with hTNF-α. (D) As in panel C except that the hMSCs were either activated hMSCs transfected with scrambled siRNA (scr) or activated hMSCs transfected with an siRNA for TSG-6. (E) As in panel D except murine macrophages cultured with zymosan with or without TSG-6. Values in panels C, D, and E are mean ± SD (n = 3; *P < .05).
As reported previously,14 hMSCs were activated by preincubation with hTNF-α to express high levels of TSG-6. Similar but variable effects were observed with preparations of hMSCs from 4 donors of bone marrow with increases of TSG-6 expression ranging from approximately 80- to 230-fold (supplemental Figure 1A). The hMSCs activated to express TSG-6 were as effective as standard preparations of hMSCs in decreasing mTNF-α expression in lavage fluid after zymosan injection (Figure 2A) and in zymosan-activated macrophages (Figure 2C). The hMSCs had no significant effect after the TSG-6 gene was knocked down with siRNA (Figure 2D). In addition, rhTSG-6 largely reproduced the effects of hMSCs in a dose-dependent manner (Figure 2E).
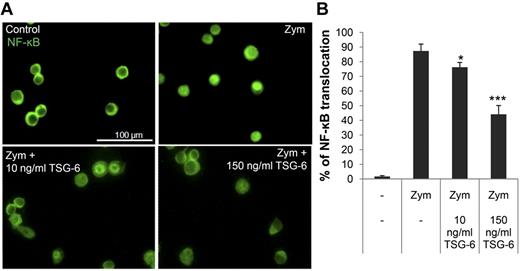
To verify that rhTSG-6 reduced NF-κB signaling in the macrophages, translocation of NF-κB from the cytoplasm to the nucleus was assayed by immunocytochemistry (Figure 3A-B). As expected, rhTSG-6 decreased the translocation of NF-κB in zymosan-stimulated macrophages in a dose-dependent manner.
TSG-6 inhibited nuclear translocation of NF-κB. Murine macrophages were incubated with zymosan with or without TSG-6. (A) Typical micrographs of immunocytochemistry are shown for cytoplasmic and nuclear distribution on NF-κB. (B) Quantification of data of micrographs from experiment in panel A. Values are mean ± SD for 3 random fields with at least 70 cells per field scored for each sample (n = 3; *P < .05; ***P < .0005).
TSG-6 inhibited nuclear translocation of NF-κB. Murine macrophages were incubated with zymosan with or without TSG-6. (A) Typical micrographs of immunocytochemistry are shown for cytoplasmic and nuclear distribution on NF-κB. (B) Quantification of data of micrographs from experiment in panel A. Values are mean ± SD for 3 random fields with at least 70 cells per field scored for each sample (n = 3; *P < .05; ***P < .0005).
hMSCs and TSG-6 reduced amplification of the pro-inflammatory signals by mesothelial cells
Although irritant-induced peritonitis is initiated by stimulation of resident macrophages,27 the pro-inflammatory signals are amplified by other cells such as the mesothelial cells that form a monolayer over all the surfaces of the peritoneum.23 To explore amplification of the inflammatory signals, we used cocultures of murine macrophages (RAW264.7) and human mesothelial cells (Met-5A). We then assayed stimulation of human mesothelial cells in the cocultures with mouse cells by real-time RT-PCR assays specific for expression of human cytokines. The mesothelial cells were not stimulated by zymosan to express the pro-inflammatory cytokine human IL-6, IL-8, or CCL2 (Figure 4A-B). However, in cocultures with murine macrophages and zymosan, human mesothelial cells were activated to express all 3 human pro-inflammatory cytokines (Figure 4A-B). The results therefore indicated that in the cocultures, macrophages were stimulated by zymosan to initiate pro-inflammatory signals that were amplified by mesothelial cells. As expected, addition of rhTSG-6 inhibited stimulation of mesothelial cells in the cocultures (Figure 4B).
Human MSCs and TSG-6 reduced amplification of the pro-inflammatory signals by mesothelial cells. (A) Human mesothelial (Mesoth) cells were incubated with zymosan and cultured alone or in cocultures with mouse macrophages (Mφ). Stimulation of the human mesothelial cells was assayed with human-specific RT-PCR assay for hIL-6. (B) As in panel A except TSG-6 was added to some of the cultures and stimulation of the human mesothelial cells was assayed with human-specific real-time RT-PCR assays for hIL-6, hIL-8, and hCCL2. (C) Systemic effects of the inflammatory cascade as indicated by plasma levels of mIL-6 assayed 8 hours after zymosan with or without subsequent infusion of hMSCs or TSG-6. Values are mean ± SD (n = 6 for HBSS, n = 3 for hMSCs and n = 4 for TSG-6; *P < .05; **P < .005; ***P < .005).
Human MSCs and TSG-6 reduced amplification of the pro-inflammatory signals by mesothelial cells. (A) Human mesothelial (Mesoth) cells were incubated with zymosan and cultured alone or in cocultures with mouse macrophages (Mφ). Stimulation of the human mesothelial cells was assayed with human-specific RT-PCR assay for hIL-6. (B) As in panel A except TSG-6 was added to some of the cultures and stimulation of the human mesothelial cells was assayed with human-specific real-time RT-PCR assays for hIL-6, hIL-8, and hCCL2. (C) Systemic effects of the inflammatory cascade as indicated by plasma levels of mIL-6 assayed 8 hours after zymosan with or without subsequent infusion of hMSCs or TSG-6. Values are mean ± SD (n = 6 for HBSS, n = 3 for hMSCs and n = 4 for TSG-6; *P < .05; **P < .005; ***P < .005).
To confirm the observations in vivo, we assayed the plasma levels of the pro-inflammatory cytokine mouse IL-6 8 hours after intraperitoneal administration of zymosan (Figure 4C). Intraperitoneal administration of hMSCs or rhTSG-6 decreased the serum levels of IL-6.
The inhibitory effects of TSG-6 were dependent on CD44
CD44 is a negative regulator of TLR2 signaling in macrophages,28 and TSG-6 was shown to modulate the interaction of hyaluronan with the cell surface receptor CD44.29 Therefore, we tested the hypothesis that the inhibitory effects of TSG-6 were dependent on the expression of CD44 on macrophages. We used 3 experimental strategies.
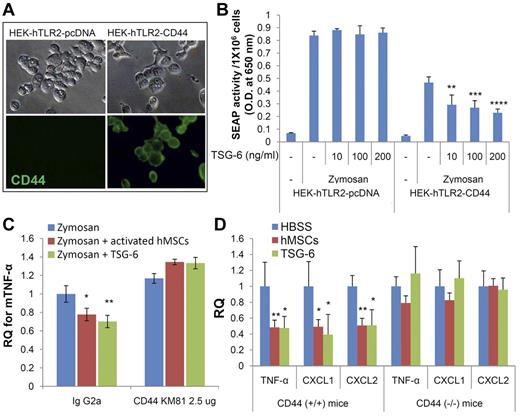
One strategy was to compare the effects of rhTSG-6 in a reporter cell line for TLR2/NF-κB signaling (HEK-Blue-hTLR2) and prepare derivatives of the line that stably expressed CD44 (Figure 5A and supplemental Figure 3). Zymosan stimulated NF-κB signaling in the line, but rhTSG-6 had no effect in the parent cell line that did not express CD44. Stable expression of CD44 in the cell line decreased the response to zymosan (Figure 5B), an observation consistent with the negative regulation of TLR2 signaling by CD44.29 As expected, rhTSG-6 decreased NF-κB signaling in the cells stably expressing CD44.
Three experimental strategies demonstrated CD44 was essential for inhibition by TSG-6. (A) Immunocytochemistry assay for expression of CD44 in clones of NF-κB reporter cells stably transfected with control plasmid (HEK-hTLR2-pcDNA) or plasmid containing complementary DNA for CD44 (HEK-hTLR2-CD44). (B) Assays for NF-κB signaling in the reporter cells expressing secreted alkaline phosphatase (SEAP). HEK-hTLR2-pcDNA and HEK-hTLR2-CD44 were incubated for 7 hours with zymosan with or without rhTSG-6. Values are mean ± SD (n = 3; **P < .001). (C) Murine macrophages pre-incubated for 15 minutes with control IgG (rat IgG2a) or blocking antibody for CD44 (CD44 KM81) and then incubated for 4 hours with zymosan and with or without activated hMSCs or TSG-6 (conditions as in Figure 2E). (D) Real-time RT-PCR assays with mouse-specific primers on resident macrophages isolated from wild-type mice (CD44+/+) and transgenic mice (CD44−/−) after injection of zymosan followed by injection of HBSS (n = 9 for CD44+/+ and n = 5 for CD44−/−), 1.6 × 106 hMSCs (n = 5 for CD44+/+ and n = 3 for CD44−/−) or 30 μg of rhTSG-6 (n = 3 for CD44+/+ and CD44−/−). Values are mean ± SD (*P < .05; **P < .005).
Three experimental strategies demonstrated CD44 was essential for inhibition by TSG-6. (A) Immunocytochemistry assay for expression of CD44 in clones of NF-κB reporter cells stably transfected with control plasmid (HEK-hTLR2-pcDNA) or plasmid containing complementary DNA for CD44 (HEK-hTLR2-CD44). (B) Assays for NF-κB signaling in the reporter cells expressing secreted alkaline phosphatase (SEAP). HEK-hTLR2-pcDNA and HEK-hTLR2-CD44 were incubated for 7 hours with zymosan with or without rhTSG-6. Values are mean ± SD (n = 3; **P < .001). (C) Murine macrophages pre-incubated for 15 minutes with control IgG (rat IgG2a) or blocking antibody for CD44 (CD44 KM81) and then incubated for 4 hours with zymosan and with or without activated hMSCs or TSG-6 (conditions as in Figure 2E). (D) Real-time RT-PCR assays with mouse-specific primers on resident macrophages isolated from wild-type mice (CD44+/+) and transgenic mice (CD44−/−) after injection of zymosan followed by injection of HBSS (n = 9 for CD44+/+ and n = 5 for CD44−/−), 1.6 × 106 hMSCs (n = 5 for CD44+/+ and n = 3 for CD44−/−) or 30 μg of rhTSG-6 (n = 3 for CD44+/+ and CD44−/−). Values are mean ± SD (*P < .05; **P < .005).
A second strategy was to use an antibody (CD44 KM81) that blocked the interaction of hyaluronan with CD44.30 As indicated in Figure 5C, the antibody negated the effects of hMSCs and rhTSG-6 on the expression of mTNF-α in zymosan-stimulated macrophages.
A third strategy was to produce zymosan-induced peritonitis in transgenic mice with inactivated alleles for CD44 and to assay the expression of pro-inflammatory cytokines in resident macrophages isolated from the peritoneum (Figure 5D). To obtain resident macrophages, the cells were isolated from peritoneal lavage fluid 2 hours after administration of zymosan and therefore before the invasion of peripheral monocytes/macrophages (Figure 1C). The cells in the lavage fluid were sorted first with magnetic beads linked to CD11b and then by FACS for F4/80 positive cells (supplemental Figure 4). The resident macrophages isolated from wild-type mice and CD44 knockout mice after administration of zymosan were activated to express the pro-inflammatory cytokines mTNF-α, mCXCL1, and mCXCL2 (Figure 5D). As expected, injection of either hMSCs or rhTSG-6 15 minutes after the zymosan decreased the levels of the mRNAs for the pro-inflammatory cytokines in the isolated wild-type resident macrophages. In contrast, neither hMSCs nor rhTSG-6 had any effect on reducing the expression of the pro-inflammatory cytokines in resident macrophages isolated from CD44−/− mice (Figure 5D).
The inhibitory effects did not involve downstream targets of TLR2 signaling
To exclude the possibility that the inhibitory effects were through downstream targets of TLR2 signaling, experiments were performed with cells over-expressing genes for 2 of the Toll-like receptor adaptor proteins that interact with the cytoplasmic tail of TLR231,32 : myeloid differentiation primary response protein 88 (MyD88) and MyD88-adapter like TIR domain-containing adapter (TIRAP). Genes for the 2 proteins were transiently transfected into the reporter cell line for NF-κB signaling that stably expressed CD44 (HEK-hTLR2-CD44). As expected, over-expression of either MyD88 or TIRAP increased zymosan-induced NF-κB signaling (supplemental Figure 5). However, rhTSG-6 did not inhibit the signaling, which indicates that TSG-6 acted upstream of the receptor adaptor proteins.
Discussion
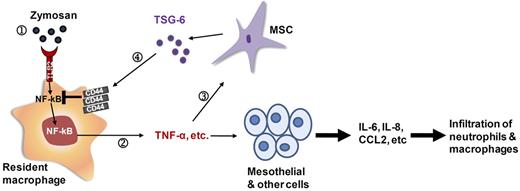
The results demonstrated that a novel mechanism whereby hMSCs or rhTSG-6 attenuated the cascade of inflammatory cytokines released by resident macrophages in zymosan-induced peritonitis. In effect, the hMSCs introduced a negative feedback loop through TSG-6 to reduce the feed-forward inflammatory response, much as suggested by previous observations with TSG-6.33,34 As indicated in Figure 6 (Step 1), zymosan bound to TLR2 on resident macrophages35 to stimulate NF-κB signaling and secretion of TNF-α and other pro-inflammatory cytokines (Step 2). The pro-inflammatory effects of the secreted cytokines were then amplified by mesothelial and probably other cells in the peritoneum. However, TNF-α and other pro-inflammatory cytokines also activated hMSCs to secrete TSG-6 (Steps 3-4). TSG-6 interacted with CD44 either directly or in complexes that did not displace hyaluronan and other ligands.24,30 The interaction of TSG-6 with CD44 was shown here for the first time to decrease zymosan/TLR2-mediated stimulation of NF-κB signaling (Step 5). The essential role of CD44 was demonstrated by: (1) the observations that rhTSG-6 did not affect zymosan-induced NF-κB signaling in a NF-κB reporter cell line until the cells were transfected to express CD44, (2) the effects were not observed in the presence of a blocking antibody to CD44, and (3) rhTSG-6 did not inhibit the synthesis of pro-inflammatory cytokines by resident macrophages from transgenic mice that did not express CD44. The inhibitory effect of TSG-6 was explained by the negative regulation of TLR2 by CD44, because rhTSG-6 did not inhibit NF-κB signaling in cells that over-expressed 2 receptor adaptor proteins (MyD88 and TIRAP) that interact with the cytoplasmic tail of TLR2 and several other TLRs.31,32
Illustration of anti-inflammatory action of hMSCs mediated mainly through TSG-6. (1) Zymosan activated macrophages via TLR2. (2) Activated NF-κB increased the expression of pro-inflammatory cytokines. (3) HMSCs were activated by the pro-inflammatory cytokines to secrete TSG-6. (4) TSG-6 negatively regulated the TLR2-mediated responses through CD44.
Illustration of anti-inflammatory action of hMSCs mediated mainly through TSG-6. (1) Zymosan activated macrophages via TLR2. (2) Activated NF-κB increased the expression of pro-inflammatory cytokines. (3) HMSCs were activated by the pro-inflammatory cytokines to secrete TSG-6. (4) TSG-6 negatively regulated the TLR2-mediated responses through CD44.
The results are consistent with the previous observations with conditionally ablated macrophages demonstrating that irritant-induced peritonitis was initiated by stimulation of resident macrophages.27 However, a different mode of action of MSCs was suggested by experiments with a cecal ligation and puncture model.36 The results indicated that MSCs attenuated sepsis by MSCs being stimulated to secrete prostaglandin E2, and the prostaglandin E2 then reprogrammed resident macrophages to increase production of IL-10.36 Other reports suggested that the anti-inflammatory activity of MSCs was explained by the cells expressing IL 1 receptor antagonist37 or the soluble TNF receptor 1.38 Therefore, it is apparent that MSCs can produce a variety of anti-inflammatory factors in addition to TSG-6.6,12 The results presented here do not rule out the effects from additional anti-inflammatory factors produced by hMSCs, but the data from the experiments with siRNA and rhTSG-6 demonstrate that TSG-6 made a major contribution to the decrease in neutrophil recruitment observed in the zymosan-induced peritonitis model.
TSG-6 was originally identified as a complementary DNA induced by incubation of human fibroblasts with TNF-α.39 There is little or no constitutive expression of TSG-6 in adult tissues, but the protein is synthesized by fibroblasts and many other cell types in response to stimulation with several pro-inflammatory mediators. Human MSCs were particularly responsive to TNF-α and expressed much higher levels than cultured fibroblasts.14 The anti-inflammatory activities of TSG-6 were demonstrated in several in vivo models and in transgenic mice with inactivation or over-expression of the gene.33 The anti-inflammatory activities were ascribed to several effects, including binding to pro-inflammatory fragments of hyaluronan, inhibiting the inflammatory cascade of proteases, and inhibiting the influx of neutrophils into sites of inflammation.40 The results presented here indicate that TSG-6 reduced the infiltration of neutrophils at least in part by attenuating the initial TLR2/NF-κB signaling in resident macrophages.
The suppressive effects of TSG-6 on NF-κB signaling in resident macrophages in the peritoneum suggest that MSCs and TSG-6 can modulate many inflammatory responses because resident macrophages play a key role in initiating the inflammation in most tissues.41 Administration of hMSCs or rhTSG-6 may have an advantage over current anti-inflammatory therapies such as anti–TNF-α agents,42,43 because the observations here indicate that MSCs attenuate the inflammatory cascade early and before high levels of the pro-inflammatory factors are generated and begin to exert systemic effects.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The work was supported in part by grants from NIH (P40 RR 17 447, P01 HL 075161 and 1 R01 HL080682).
National Institutes of Health
Authorship
Contribution: H.C. and R.H.L. designed research, performed experiments, analyzed data, and wrote the paper; N.B. performed experiments; J.Y.O. analyzed data; and D.J.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: D.J.P. is a member of the scientific advisory board of Temple Therapeutics LLC. The remaining authors declare no competing financial interests.
Correspondence: Darwin J. Prockop, Texas A&M Health Science Center, College of Medicine, Institute for Regenerative Medicine at Scott & White, Temple, TX 76502; e-mail: Prockop@medicine.tamhsc.edu.
References
Author notes
H.C. and R.H.L. made equal contributions to this work.