In this issue of Blood, Laurent et al use 3-D confocal imaging to visualize CD8+ T-cell lytic immune synapses in follicular lymphoma and report that this activity may influence progression-free survival after rituximab-chemotherapy.1
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma (NHL), accounting for 25% of newly diagnosed cases, and is characterized by the proliferation of CD20+ tumor B cells within lymph node follicles. The introduction of the chimeric anti-CD20 monoclonal antibody rituximab has revolutionized the treatment of this lymphoma. In particular, the combination of rituximab and chemotherapy (R-chemo) with subsequent rituximab maintenance has greatly improved response rates, progression-free survival (PFS), and overall survival in FL. This regimen is now the gold standard for both first-line induction treatment and relapsed advanced disease. Rituximab represents a major cornerstone of immunotherapy and places FL at the forefront of cancer immunotherapy. However, in common with other cancers, disease relapse and therapy resistance remain major clinical problems, and FL is still regarded as an incurable disease. Thus, it is clear that new targets and biomarkers are needed to improve therapeutic strategies.
The tumor microenvironment is currently thought to play a significant role in cancer biology,2 and its characterization, including the complex signaling interactions between tumor cells and nonmalignant cells, should allow a better scientific understanding of disease pathophysiology. FL is a good model to study the role of the microenvironment, with extensive gene-expression profiling and immunohistochemistry (IHC) studies providing evidence that a high content of T cells is prognostically favorable, while pro-tumor macrophages may confer an unfavorable clinical course.3 Another important variable is the ability of tumor cells to actively manipulate surrounding nonmalignant stromal and immune cells to create a pro-tumor environmental niche. For example, FL cell–immune cell direct contact interactions have been shown to drive chronic CD4+ T-cell cytokine release resulting in tumor cell growth and survival axis,4 while at the same time suppressing CD8+ and CD4+ T-cell actin cytoskeletal signaling complexes at the immunologic synapse.5 These synapses are essential for generating effective host anti-tumor responses. This dual subversion of T cells together with other immunomodulatory mechanisms likely contributes to FL disease progression and also helps explain the effectiveness of therapeutic regimens including R-chemo.
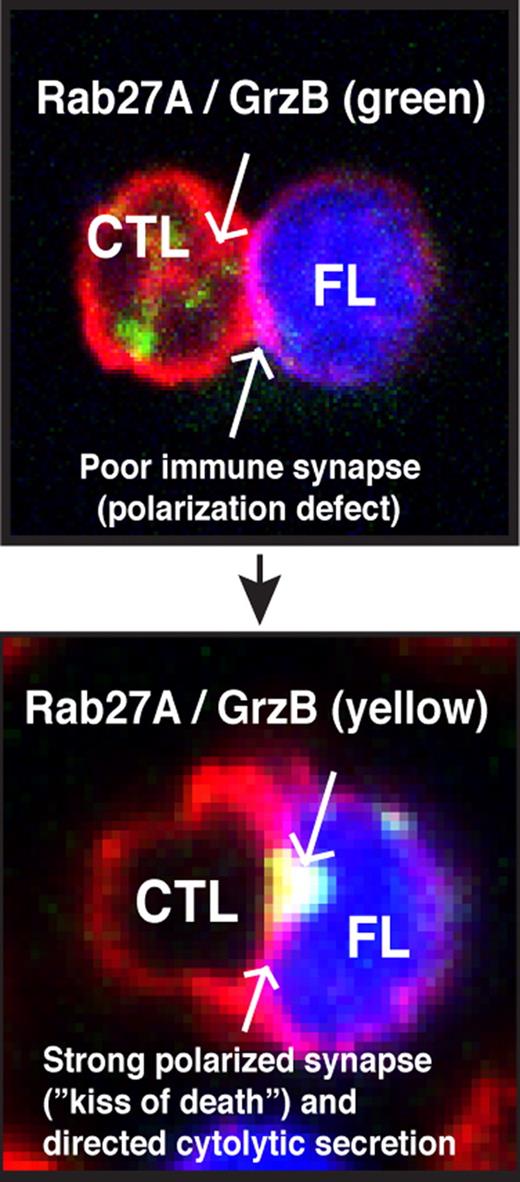
This article by Laurent et al has very elegantly combined IHC and advanced confocal microscopy technology to provide strong evidence that CD8+ cytotoxic T lymphocytes (CTLs) play an active role in the FL microenvironment. Importantly, the authors identify granzyme B+ (GrzB) as a potential activation biomarker for CTL functional status in FL. Activated CTLs that encounter tumor cells expressing cognate antigen will rapidly form an F-actin–rich immune synapse with target cells. This lytic synapse or “kiss of death” produces anti-tumor activity by the directed secretion of GrzB protease into target cells to induce cell death. Immunohistochemistry analysis revealed a high infiltrate of GrzB+ CD8+ T cells in an FL interfollicular location. Examining both the number of positive cells and the intensity of GrzB staining allowed calculation of a biomarker score for each FL patient examined and definition of 2 patient groups: low and high GrzB staining. Moreover, 3-color confocal microscopy was used to identify increased numbers of these cells including GrzB expression in FL compared with nonmalignant counterpart reactive lymph node tissue. The authors then used 3-D image reconstructions to allow, for the first time, the in situ visualization of CTL lytic immune synapse structures with FL tumor cells. Interestingly, these effector cells did not enter the follicle but were mostly located within the interfollicular space around nodules. The co-staining of activated capsase-3 on FL cells that formed cell conjugates with CTLs provides strong evidence for cytotoxic function. This was verified by performing functional cytotoxicity assays using expanded tumor-infiltrated CD8+ T cells (TILs) from FL patients against autologous FL cells pulsed with superantigen (in vitro immunologic assay that stimulates a large proportion of T cells triggering antigen-induced T-cell receptor signaling and effector function). Of interest, this study also reveals that high GrzB expression in CTLs (high GrzB score patient group) correlates with prolonged PFS in FL patients treated with R-chemo compared with the low GrzB score patient cohort.
To date, the results of many IHC studies investigating the role of the immune microenvironment in FL have been contradictory with technical scoring variations between laboratories acknowledged as a potential issue. Laurent et al have overcome this challenge by combining IHC with confocal microscopy, 3-D reconstructed image analysis, and functional cytotoxicity assays. This immunologic approach has identified strong GrzB expression as a biomarker for activated CTLs capable of forming lytic immune synapses and mounting cytotoxic function against autologous tumor cells. There are, however, some important considerations and future study is required to identify biomarkers and targets for enhancing the clinical potential of immunotherapy. Laurent and colleagues al show that autologous CTLs exhibiting a strong GrzB content did not enter the FL nodule. It is possible that a cancer-associated T-cell motility defect or tumor “barrier” may prevent CTLs from infiltrating the intrafollicular tumor site in FL. Moreover, work using purified TILs, confocal imaging, and quantitative image analysis has identified that it is not only expression levels of biomarkers that is important, but also critical whether these proteins can traffic and polarize to the synapse effector site. FL tumor cells were shown to rapidly induce failure of recruitment of cytoskeletal signaling proteins including cytotoxic machinery (Rab27A) to the synapse site in previously healthy CTLs.5 This profound tumor-induced T-cell immune synapse defect in FL could be repaired by the immunomodulatory drug lenalidomide. The high-quality confocal images presented by Laurent et al suggest that TILs may exhibit nonpolarized GrzB trafficking defects to the synapse contact site compared with nontumor-exposed autologous peripheral blood T cells from FL patients. Clearly, translational researchers must consider identifying strategies to repair tumor-induced CTL cell defects to address the unmet clinical needs in FL and maximize the anti-tumor activity of immunotherapy regimens such as R-chemo. This has been highlighted in CLL with strikingly impressive clinical results after infusion of genetically modified autologous T cells targeting tumor cells.6 The challenge now is, can CTL activity be enhanced or re-engineered by immunomodulatory strategies to tilt the balance away from immunosuppression in the microenvironment and toward potent anti-tumor activity (see figure) and maximizing the kiss of death in FL?
Summary of functional CD8+ T cell (CTL) activity in FL. CTLs have the potential to be powerful effector cells in the treatment of FL. The identification of therapeutic agents or strategies that target and enhance their activity may address the unmet clinical needs in follicular lymphoma (FL). The identification of granzyme B (GrzB) as a powerful biomarker for lytic immune synapse function should aid this translational research goal.
Summary of functional CD8+ T cell (CTL) activity in FL. CTLs have the potential to be powerful effector cells in the treatment of FL. The identification of therapeutic agents or strategies that target and enhance their activity may address the unmet clinical needs in follicular lymphoma (FL). The identification of granzyme B (GrzB) as a powerful biomarker for lytic immune synapse function should aid this translational research goal.
Conflict-of-interest disclosure: A.G.R. declares no competing financial interests. J.G.G. has received honoraria from Roche, Celgene, and Merck for work on advisory boards. ■
REFERENCES
National Institutes of Health