Abstract
c-Cbl protein functions as an E3 ligase and scaffolding protein, where 3 residues, Y700, Y731, and Y774, upon phosphorylation, have been shown to initiate several signaling cascades. In this study, we investigated the role of these phospho-tyrosine residues in the platelet functional responses after integrin engagement. We observed that c-Cbl Y700, Y731 and Y774 undergo phosphorylation upon platelet adhesion to immobilized fibrinogen, which was inhibited in the presence of PP2, a pan-src family kinase (SFK) inhibitor, suggesting that c-Cbl is phosphorylated downstream of SFKs. However, OXSI-2, a Syk inhibitor, significantly reduced c-Cbl phosphorylation at residues Y774 and Y700, without affecting Y731 phosphorylation. Interestingly, PP2 inhibited both platelet-spreading on fibrinogen as well as clot retraction, whereas OXSI-2 blocked only platelet-spreading, suggesting a differential role of these tyrosine residues. The physiologic role of c-Cbl and Y731 was studied using platelets from c-Cbl KO and c-CblYF/YF knock-in mice. c-Cbl KO and c-CblYF/YF platelets had a significantly reduced spreading over immobilized fibrinogen. Furthermore, clot retraction with c-Cbl KO and c-CblYF/YF platelets was drastically delayed. These results indicate that c-Cbl and particularly its phosphorylated residue Y731 plays an important role in platelet outside-in signaling contributing to platelet-spreading and clot retraction.
Introduction
Platelet outside-in signaling is an important process in hemostasis and refers to the process of fibrinogen binding to αIIbβ3, an integrin only expressed in platelets and megakaryocytes. Upon fibrinogen binding, integrin αIIbβ3 undergoes a conformational change that initiates signaling cascades that modulate the actin cytoskeleton leading to cell spreading, filopodia and lamellipodia formation and at later stages, cell retraction.1 Coordinated spreading and retraction is of pivotal importance in platelets as stable platelet adhesion and thrombus formation depends on it. Engagement of αIIbβ3 results in tyrosine phosphorylation of proteins, therefore the role of tyrosine kinases in platelet outside-in signaling has been extensively studied. A hierarchical activation of Src-family kinases (SFK) and Spleen Tyrosine kinase (Syk) has been proposed as a general mechanism for integrin signaling.2 Of the members of SFK expressed in platelets both Src and Fyn have been reported to interact with β3 cytoplasmic tail although at distinct sites and with different functional consequences. Src constitutively associates with the RGT sequence on the β3 tail,3 becomes activated upon fibrinogen binding and its further removed by a calpain-dependent cleavage.1 Fyn associates with the IHDRK sequence on the β3 tail, has a sustained interaction with the integrin through out the platelet activation process, and is postulated as an alternative pathway to support platelet-spreading.4 Syk, on the other hand, is tyrosine phosphorylated in a SFK-dependent manner.5,6 The activation of Src kinase and Syk by αIIbβ3 results in tyrosine phosphorylation of signaling molecules and adaptor proteins that are implicated in modulation of the actin cytoskeleton.7,8 Recently, the involvement of ITAM motifs of the FcγRIIA receptor has also been described in the human platelet outside-in signaling complex,9 which suggests the possibility that integrins signal as immunoreceptors. In this context, the activation of Syk by αIIbβ3 is mediated by the ITAM motif of FcγRIIA.
c-Cbl and Cbl-b are members of the Cbl mammalian family of proteins that are expressed in hematopoietic cells. Because of their E3 ubiquitin ligase activity they have been implicated as important negative regulator of signaling events, particularly downstream of tyrosine kinases.10 Another important function of Cbl proteins is to act as adaptor molecules; they bear a proline-rich region which mediates binding to SH3 domain-containing proteins and on the C-terminal region, Cbl proteins contain several tyrosine phosphorylation sites that regulate their interaction with SH2 domains.11,12 Among these phosphorylation sites, tyrosine residue 700 (Y700) in c-Cbl has been described as docking site for Vav13 while tyrosine residue 774 (Y774) is responsible for Crk/CrkL binding.14,15 Importantly, tyrosine 731 (Y731 in humans; Y737 in mouse) which is unique to c-Cbl, has been shown to bind to the SH2 domain of the p85 subunit of PI3K promoting the assembly of a protein complex that regulates PI3K function.16,17 Tyrosine phosphorylation of c-Cbl has been implicated in many signaling pathways. Receptors that lead to c-Cbl phosphorylation includes various Fcγ (eg, T-cell receptor/CD3, platelet collagen receptor GPVI), growth factor (eg, epidermal growth factor receptor), hormones (eg, prolactin receptor) and cytokine receptors (eg, thrombopoietin receptor).11 Integrin β1, β2, and β3 cross linking has also been linked with c-Cbl phosphorylation18,19 generating binding sites for molecules that modulate the actin cytoskeleton.20,21
Platelets express c-Cbl22 but its role is still not clearly defined. Previous work from our group23 and others24 have identified c-Cbl as an important regulator of GPVI-mediated signaling. However, the role of c-Cbl in integrin-mediated outside-in signaling in platelets is not completely understood. Miranti et al8 showed the involvement of c-Cbl downstream of αIIbβ3 signaling in the A5 cell line, which stably expresses αIIbβ3. Another study showed that after αIIbβ3 engagement, c-Cbl is tyrosine phosphorylated and recruits PI3K25 but the mechanism and implications of this activation or the downstream targets remain to be elucidated.
Given the importance of c-Cbl regulation in integrin signaling in other hematopoietic cell types,6,18,20,26 we have investigated the mechanism of c-Cbl tyrosine phosphorylation in human platelets and its role in platelet outside-in signaling using platelets from c-Cbl KO mice and c-CblYF/YF knock-in mice, in which the tyrosine residue 737 (murine equivalent of human Y731) was mutated to phenylalanine.27,28 Here we report that platelet outside-in signaling causes c-Cbl phosphorylation on Y700, Y731, and Y774. Phosphorylation on residue Y731 is differentially regulated by SFK, whereas Y700 and Y774 are mostly phosphorylated in a Syk-dependent manner. c-Cbl KO and c-CblYF/YF platelets had a reduced spreading and clot retraction was significantly delayed. Our results describe a novel role for c-Cbl in platelet outside-in signaling.
Methods
Materials
All reagents were obtained from Sigma-Aldrich unless stated otherwise. Anti–phospho-c-Cbl Y731, Y774, and SFK-phospho Y416 were purchased from Cell Signaling Technology. Anti–phospho-c-Cbl Y700, anti–c-Cbl, CD41-PE, JON/A-PE and P-selectin-FITC antibodies were purchased from BD Biosciences. Anti-phosphotyrosine antibody 4G10 antibody was from Millipore. PP2 and PP3 were from Enzo Life Sciences. AYPGKF was custom synthesized at Invitrogen. Spleen Tyrosine Kinase Inhibitor, 3-(1-Methyl-1H-indol-3-yl-methylene)-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide (OXSI-2) was obtained from Calbiochem. Human fibrinogen (plasminogen, VWF and Fibronectin depleted) was from Enzyme Research Laboratories. All reagents were analytical grade.
Mice
Studies with human platelets
Platelets were isolated from freshly drawn venous blood, collected from healthy volunteers with informed consent and aggregation studies were performed as previously described.29 The donors claimed not to have taken any medication during 2 weeks before blood collection. Platelet count was adjusted to of 3 × 108 platelets/mL for spreading experiments and 2 × 108 platelets/mL for aggregation studies.
Studies with mouse platelets
Blood was collected from the vena cava of anesthetized mice as previously described.29 The platelet pellet was resuspended in Tyrode buffer (pH 7.4) containing 0.2 unit/mL apyrase and 10μM indomethacin. Platelet counts were determined using a Hemavet 950FS blood cell counter (Drew Scientific Inc). For aggregation studies a density of 2 × 108 platelets/mL was used. Flow cytometry was performed as previously described.30
Protein tyrosine phosphorylation
Platelet adhesion to fibrinogen: 100 mm bacterial tissue culture plates were precoated with 100 μg/mL fibrinogen.31 After blocking with heat-denatured BSA, 3 × 108 platelets in 2.0 mL were added and incubated for the indicated periods of time at 37°C in a CO2 incubator. Platelets adherent to fibrinogen were gently rinsed twice with PBS, reaction was stopped by the addition of cold lysis buffer (150mM NaCl, 25mM Tris [pH 7.6], 1% Nonidet P-40, 2mM EDTA, 2mM EGTA, 1mM sodium orthovanadate, 10mM sodium fluoride, and 10 μg/mL leupeptin). After protein determination (BCA protein assay kit, Thermo Scientific), 40-80 μg of total cell lysate was electrophoresed on 8% SDS-PAGE gels. Proteins were transferred to a PDVF membrane (Millipore Corp), subjected to Western blotting,30 blocked for 1 hour with odyssey blocking buffer and incubated with the appropriate antibody 1:500 (vol/vol) in odyssey blocking buffer with 0.1% (vol/vol) Tween 20 (16 hours, 4°C). After washing, membranes were incubated with the appropriate LICOR secondary antibody (1 hour, 4°C). Phosphorylation of proteins was visualized and quantified using the Odyssey system.
In some experiments platelets were treated with the SFK inhibitor PP2 (10μM), its negative analog PP3 (10μM) or with the Syk inhibitor OXSI-2 (2μM) 10 minutes before plating them over the fibrinogen coated surface.
Microscopy
Washed human or murine platelets (1 × 106 platelets/mL) were plated on fibrinogen-coated cover slips for the indicated time point at 37°C in a CO2 incubator. When indicated, platelets were treated with PP2 (10μM), PP3 (10μM) or OXSI-2 (2μM). Adherent cells were fixed with 3.7% paraformaldehyde for 20 minutes. For confocal microscopy, platelets were permeabilized with 0.1% Triton X-100. Rhodamine-phalloidin (Invitrogen) was used to stain F-actin. Platelets were examined with a confocal system (LEICA TCS FP5 X). Images were processed in Adobe Photoshop. Surface areas of at least 20 platelets per experiment were measured using ImageJ 1.440 software. In other set of experiments human or murine platelets were analyzed by differential interference contrast (DIC) using a Nikon Eclipse TE300 with a digital camera DXM1200 (Nikon Instruments Inc).
Clot retraction
Human or murine platelets were isolated as previously described. A suspension of 500 μL for human platelets and 100 μL of murine platelets at a concentration of 3 × 108 platelets/mL was added to a glass cuvette, mixed with 1mM CaCl2 and 0.2-0.4 units/mL of thrombin. In some experiments, exogenous fibrinogen was added to a final concentration of 0.1 mg/mL. Platelets were allowed to retract and photographed at the indicated time points.
Statistical analysis
Each experiment was repeated at least 3 times. Results are expressed as means ± SEM with number of observations n. Data were analyzed using GraphPad Prism 5 software. Significant differences were determined using Student t test. Differences were considered significant at P ≤ .05.
Results
c-Cbl is tyrosine phosphorylated in human platelet outside-in signaling
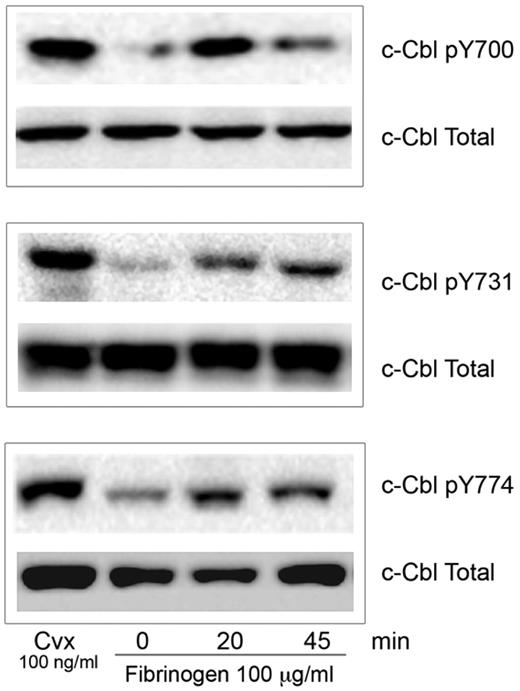
The role of phosphorylated c-Cbl protein downstream of integrin αIIbβ3 is still poorly understood. To identify the specific tyrosine residues on c-Cbl that are phosphorylated during integrin mediated signaling, we allowed human platelets to spread over a fibrinogen-coated surface. Western blot analysis was performed using phospho-specific antibodies that detect tyrosine phosphorylation sites Y700, Y731, and Y774.12 As a robust c-Cbl tyrosine phosphorylation downstream of GPVI has been previously described23,24 we used convulxin (Cvx) treated platelets as a positive control. c-Cbl tyrosine phosphorylation occurred by 20 minutes and it was sustained until 45 minutes (Figure 1). This result indicates that c-Cbl is phosphorylated on all 3 tyrosine residues during platelet outside-in signaling.
c-Cbl activation downstream of αIIbβ3. Human platelets (3 × 108 platelets/mL) were plated over fibrinogen (100 μg/mL) coated plates for the indicated time points. Reaction was stopped with cold lysis buffer. Lysates were examined for c-Cbl phosphorylation using phosphospecific antibodies for residues Y700, Y731 and Y774 as indicated. Equal protein loading was detected with anti–c-Cbl antibody. Cells were also treated with convulxin (Cvx) to ascertain tyrosine phosphorylation of Cbl (positive control). All blots are representative of 3 experiments.
c-Cbl activation downstream of αIIbβ3. Human platelets (3 × 108 platelets/mL) were plated over fibrinogen (100 μg/mL) coated plates for the indicated time points. Reaction was stopped with cold lysis buffer. Lysates were examined for c-Cbl phosphorylation using phosphospecific antibodies for residues Y700, Y731 and Y774 as indicated. Equal protein loading was detected with anti–c-Cbl antibody. Cells were also treated with convulxin (Cvx) to ascertain tyrosine phosphorylation of Cbl (positive control). All blots are representative of 3 experiments.
Regulation of c-Cbl phosphorylation by tyrosine kinases
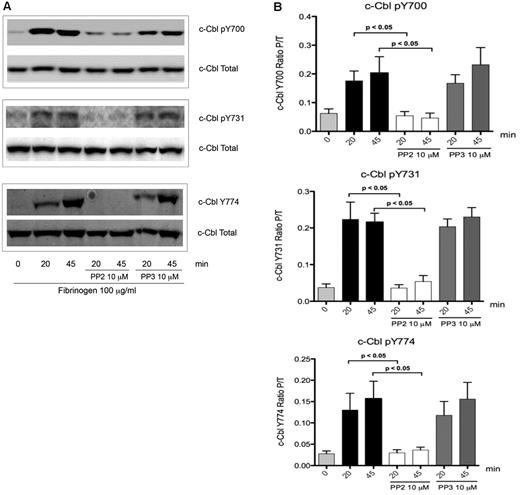
To elucidate how c-Cbl tyrosine phosphorylation is regulated downstream of integrin signaling in platelets, inhibitor studies were performed. Treatment of platelets with the SFK inhibitor PP2, abolished tyrosine phosphorylation of the residues Y700, Y731, and Y774 on c-Cbl (Figure 2A), while PP3 (negative control) had no effect. Densitometry analysis showed that PP2 inhibition is significant when compared with platelets with no treatment (Figure 2B) indicating that integrin activation results in SFK-dependent c-Cbl phosphorylation.
Role of SFKs over c-Cbl phosphorylation. (A) To determine the role of SFKs over c-Cbl phosphorylation downstream of αIIbβ3, platelets were incubated for 10 minutes with 10μM of Pan SFKs inhibitor PP2 or its negative control PP3 and then plated over fibrinogen coated plates for the indicated time points. Lysates were analyzed as described on legend from Figure 1. (B) Densitometry analysis of 5 experiments. Bars represent mean ± SEM of the ratio of phosphorylated protein over total protein. Student t test performed (P < .05, no treatment vs PP2).
Role of SFKs over c-Cbl phosphorylation. (A) To determine the role of SFKs over c-Cbl phosphorylation downstream of αIIbβ3, platelets were incubated for 10 minutes with 10μM of Pan SFKs inhibitor PP2 or its negative control PP3 and then plated over fibrinogen coated plates for the indicated time points. Lysates were analyzed as described on legend from Figure 1. (B) Densitometry analysis of 5 experiments. Bars represent mean ± SEM of the ratio of phosphorylated protein over total protein. Student t test performed (P < .05, no treatment vs PP2).
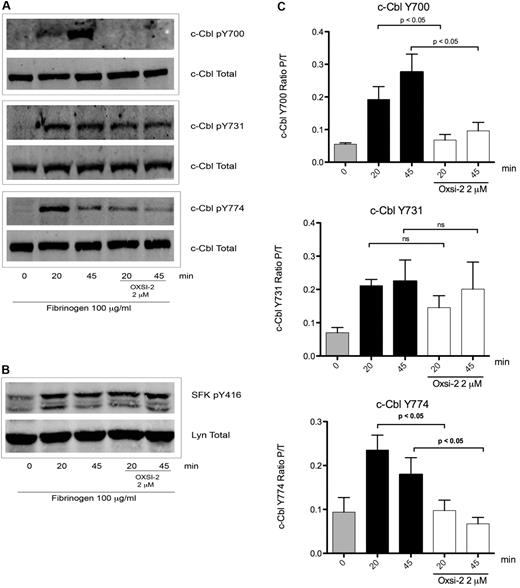
The results shown in Figure 2 do not identify the role of Syk in c-Cbl phosphorylation, since Syk kinase activation is also dependent on SFK's activity.2,32 To elucidate the role of Syk, platelets were treated with OXSI-2, a know Syk inhibitor.33 OXSI-2 caused inhibition of c-Cbl Y700 and Y774 phosphorylation (Figure 3A) suggesting that these tyrosines are phosphorylated in a Syk-dependent manner. Interestingly, phosphorylation of c-Cbl Y731 was not affected by Syk inhibition (Figure 3A middle panel). To ascertain that OXSI-2 was not affecting SFKs activity, phosphorylation of Y416 (marker of SFKs activation) was examined under the same experimental conditions. As demonstrated in Figure 3B, SFK activity downstream of αIIbβ3 was intact when the Syk inhibitor OXSI-2 was used at a concentration of 2μM. Densitometry analysis revealed that the decreased phosphorylation seen on Y700 and Y774 is significantly different compared with untreated platelets, while OXSI-2 has no significant effect over the phosphorylation of residue Y731 (Figure 3C).
Effect of Syk inhibition over c-Cbl phosphorylation downstream of αIIbβ3. (A) To determine the role of Syk mediated phosphorylation downstream of αIIbβ3 platelets were incubated for 10 minutes with 2μM of Syk inhibitor OXSI-2 and then plated over fibrinogen coated plates for the indicated time points. Lysates were analyzed as on legend from Figure 1. (B) SFKs p-Y416 was also analyzed to confirm its intact activity under the same experimental conditions. (C) Densitometry analysis of 5 experiments. Bars represent mean ± SEM of the ratio of phosphorylated protein over total protein. Student t test performed (P < .05, no treatment vs OXSI-2 for residues Y700 and Y774; ns: non-significant for residue Y731).
Effect of Syk inhibition over c-Cbl phosphorylation downstream of αIIbβ3. (A) To determine the role of Syk mediated phosphorylation downstream of αIIbβ3 platelets were incubated for 10 minutes with 2μM of Syk inhibitor OXSI-2 and then plated over fibrinogen coated plates for the indicated time points. Lysates were analyzed as on legend from Figure 1. (B) SFKs p-Y416 was also analyzed to confirm its intact activity under the same experimental conditions. (C) Densitometry analysis of 5 experiments. Bars represent mean ± SEM of the ratio of phosphorylated protein over total protein. Student t test performed (P < .05, no treatment vs OXSI-2 for residues Y700 and Y774; ns: non-significant for residue Y731).
Together, these results established that fibrinogen binding to αIIbβ3 results in SFK activation leading to c-Cbl Y731 phosphorylation and subsequent Syk-dependent phosphorylation of c-Cbl residues Y700 and Y774.
Functional effect of c-Cbl tyrosine phosphorylation on outside-in signaling-mediated events in human platelets
At the cellular level, integrin outside-in signaling regulates functional responses such as cell spreading and cell retraction. To better understand the functional outcome of c-Cbl tyrosine phosphorylation we investigated whether spreading over fibrinogen and clot retraction are affected by inhibition of the kinases responsible for c-Cbl phosphorylation and correlated these results with c-Cbl function. As shown in Figure 4A, SFK or Syk inhibition results in decreased platelet-spreading, suggesting that among other events, c-Cbl phosphorylation on residues Y700, Y731, and Y774 may be important for platelet-spreading. In contrast to the spreading experiments, clot retraction was only affected when platelets were treated with PP2 whereas Syk inhibition had no effect (Figure 4B). It is important to note that the clot retraction results with PP2 are different from those reported by Flevaris et al,1 wherein the inhibition of SFKs with the same concentration of PP2 (10μM) resulted in enhanced clot retraction. To rule out the possibility that platelet treated with PP2 had any secretion defect or that the concentration of fibrinogen present in our preparation was not enough to sustain retraction, exogenous fibrinogen (0.1 mg/mL) was added, however, even under these conditions clot retraction did not occur (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, it is possible that the differences in results are because of other components present on platelet rich plasma that are not contained in our washed platelet system.
Functional effect of c-Cbl tyrosine phosphorylation on outside-in signaling-mediated events in human platelets. (A) Human platelets (1 × 106 platelets/mL) were treated with PP2 (10μM) or OXSI-2 (2μM) for 10 minutes before be plated on fibrinogen-coated cover slips for the indicated time point. After 3 times washing with PBS, adherent cells were fixed with 3.7% paraformaldehyde and analyzed with DIC imaging. (B) Clot retraction analysis of human platelets treated with PP2 and OXSI-2.
Functional effect of c-Cbl tyrosine phosphorylation on outside-in signaling-mediated events in human platelets. (A) Human platelets (1 × 106 platelets/mL) were treated with PP2 (10μM) or OXSI-2 (2μM) for 10 minutes before be plated on fibrinogen-coated cover slips for the indicated time point. After 3 times washing with PBS, adherent cells were fixed with 3.7% paraformaldehyde and analyzed with DIC imaging. (B) Clot retraction analysis of human platelets treated with PP2 and OXSI-2.
As clot retraction occurs after thrombin stimulation, we evaluated whether c-Cbl phosphorylation occurs by GPCR signaling using a high dose of AYPGKF (500μM) as an agonist. Upon stimulation, phosphorylation of the 3 c-Cbl tyrosine residues occurs only after 60 seconds of activation, time frame in which outside-in signaling is occurring (supplemental Figure 4). Furthermore, when the fibrinogen receptor antagonist SC 57 101 was used, the phosphorylation of residue Y700, Y731, and Y774 was dramatically decreased. This result indicates that GPCR activation does not contribute to the clot retraction process through c-Cbl.
Collectively, our results indicate that Syk kinase activity, and therefore c-Cbl Y700 and Y774 phosphorylation does not appear to have a major role in clot retraction, whereas SFK-dependent c-Cbl Y731 phosphorylation correlates with both cell spreading and clot retraction.
Physiologic significance of c-Cbl tyrosine phosphorylation in platelets
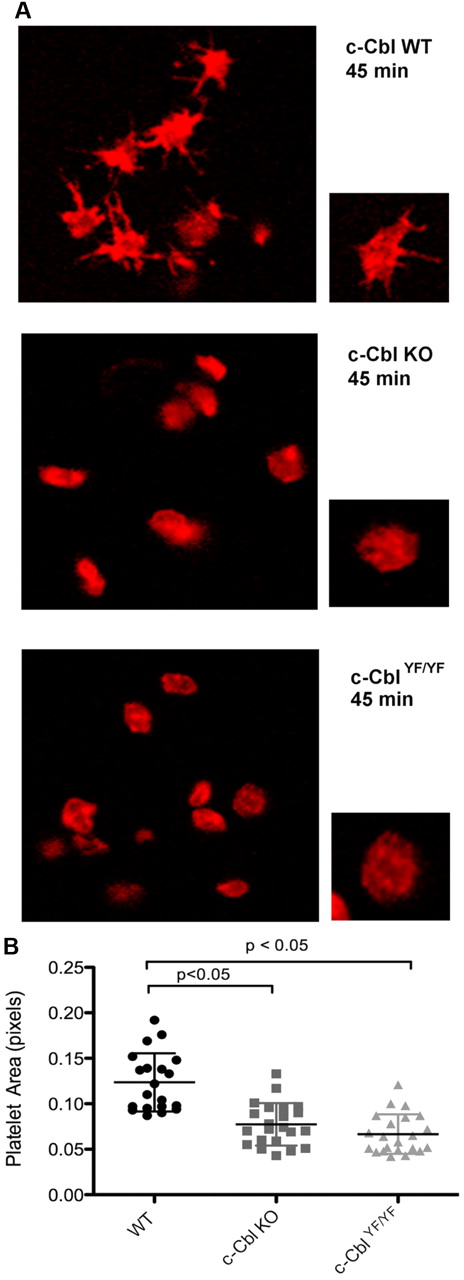
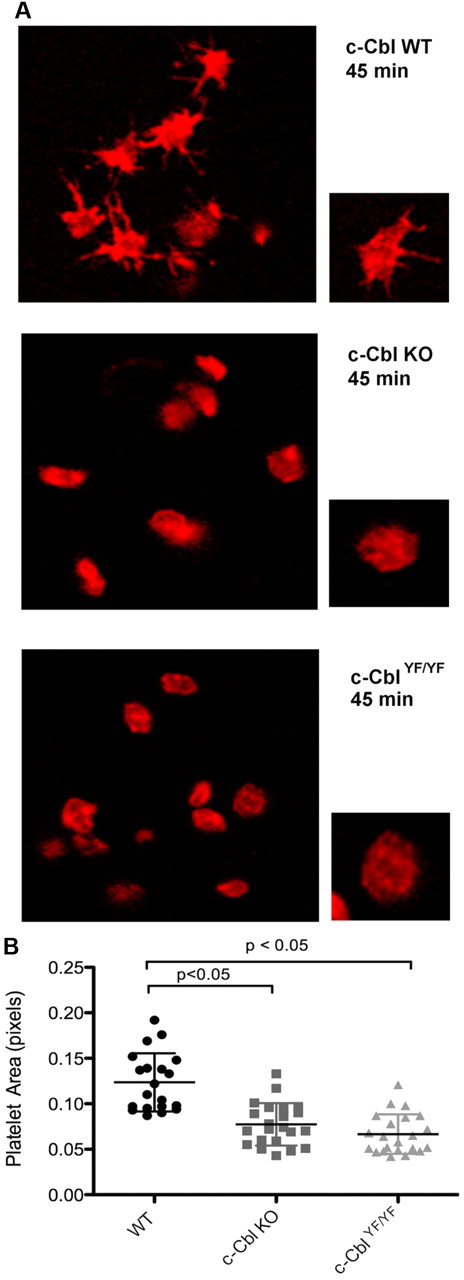
c-Cbl phosphotyrosine residues Y700, Y731, and Y774 are known to interact with the SH2-domain containing proteins Vav, PI3K, and Crk/CrkL, respectively.13,15-17 Based on these known interactions and the results shown, we investigated the physiologic consequence of c-Cbl tyrosine phosphorylation in platelet functional responses mediated by outside-in signaling. Of the 3 tyrosine residues, Y700, Y731, and Y774, we have obtained a knock-in mouse c-CblYF/YF, in which Y737 (equivalent of Y731 in human c-Cbl) was mutated to F737.27 We examined integrin-mediated spreading by performing all studies in the presence of apyrase and indomethacin to eliminate the contribution of any secreted ADP and generated thromboxane A2. We evaluated the role of c-Cbl and particularly of residue Y737 by comparing platelet-spreading on a fibrinogen-coated surface in platelets from WT, c-Cbl KO, and c-CblYF/YF mice. After fixation, platelets were stained with Rhodamine-phalloidin and analyzed by confocal microscopy. Figure 5A shows that in contrast to WT platelets, platelets from c-Cbl KO, or c-CblYF/YF mice exhibited very few filopodia formations and partly reduced spreading. Quantification of the area of at least 20 platelets per experiment, confirmed that there was a significant difference between the total areas of platelets from c-Cbl KO or c-CblYF/YF mice compared with WT mice (Figure 5B). The total platelet area of mutant platelets was significantly different from WT platelets (WT = 0.122 ± 0.03 pixels; c-Cbl KO = 0.07 ± 0.02 pixels; c-CblYF/YF = 0.06 ± 0.02 pixels). The similarity of reduced spreading observed between c-Cbl KO and c-CblYF/YF indicates that c-Cbl function in outside-in signaling is largely defined by Y737.
Role of c-Cbl Y731 on platelet-spreading. (A) Platelets from WT, c-Cbl KO, or c-CblYF/YF were treated with apyrase (0.2 unit/mL) and indomethacin (10μM) and allowed to spread over a fibrinogen-coated cover slip for the indicated time point. After fixation with 3.7% paraformaldehyde, platelets were permeabilized with 0.1% Triton X-100 and stained with Rhodamin-palloidin (red). Platelet morphology was analyzed using confocal microscopy. (B) Surface area quantitation of at least 20 platelets. Graph represents mean ± SEM of platelet area. Student t test performed (P < .05, WT vs c-Cbl KO and WT vs c-CblYF/YF). Representative of 3 different experiments.
Role of c-Cbl Y731 on platelet-spreading. (A) Platelets from WT, c-Cbl KO, or c-CblYF/YF were treated with apyrase (0.2 unit/mL) and indomethacin (10μM) and allowed to spread over a fibrinogen-coated cover slip for the indicated time point. After fixation with 3.7% paraformaldehyde, platelets were permeabilized with 0.1% Triton X-100 and stained with Rhodamin-palloidin (red). Platelet morphology was analyzed using confocal microscopy. (B) Surface area quantitation of at least 20 platelets. Graph represents mean ± SEM of platelet area. Student t test performed (P < .05, WT vs c-Cbl KO and WT vs c-CblYF/YF). Representative of 3 different experiments.
To address the question of whether the mutation on residue Y737 affects the activity of the kinases responsible for c-Cbl phosphorylation, we analyzed the general phospho-tyrosine pattern as well was the activation state of SFK and Syk by Western blotting. Comparable levels of total phosphorylation and activation of SFK or Syk was observed between WT and the c-CblYF/YF platelets (supplemental Figures 3A-C)
To out rule the possibility that the spreading defect is because of a global effect of c-Cbl gene ablation or mutation on the expression of αIIbβ3 and/or activation, expression levels of αIIb (CD41) on the surface of platelets from WT, c-Cbl KO, and c-CblYF/YF mice were examined using flow cytometry. No significant difference was observed among the 3 genotypes (Figure 6A). A similar approach was taken to analyze the AYPGKF-induced integrin activation (Jon/A binding) and platelet secretion (P-selectin). No significant difference was observed (Figure 6B-C) suggesting that there is no integrin activation or secretion abnormality on c-Cbl KO or c-CblYF/YF platelets downstream of protease-activated receptors. Collectively, these results clearly indicate that the reduced spreading in c-Cbl KO and c-CblYF/YF platelets is inherent to integrin-mediated signaling toward the cytoskeleton.
CD41 expression, integrin αIIbβ3 activation and α granule secretion on c-Cbl KO and c-Cbl YF/YF platelets. (A) Washed platelets from WT, c-Cbl KO and c-CblYF/YF mice were analyzed for αIIb expression with CD-41–FITC antibody. (B) WT, c-Cbl KO and c-CblYF/YF were stimulated with the indicated dose of PAR 4 agonist AYPYKF for 15 minutes in the presence of FITC-labeled anti–P-selectin (CD62) to test α-granule secretion or with PE-labeled JON/A antibody to evaluate integrin αIIbβ3 activation (C). Graphs represent mean ± SEM of % positive cells of 3 different experiments.
CD41 expression, integrin αIIbβ3 activation and α granule secretion on c-Cbl KO and c-Cbl YF/YF platelets. (A) Washed platelets from WT, c-Cbl KO and c-CblYF/YF mice were analyzed for αIIb expression with CD-41–FITC antibody. (B) WT, c-Cbl KO and c-CblYF/YF were stimulated with the indicated dose of PAR 4 agonist AYPYKF for 15 minutes in the presence of FITC-labeled anti–P-selectin (CD62) to test α-granule secretion or with PE-labeled JON/A antibody to evaluate integrin αIIbβ3 activation (C). Graphs represent mean ± SEM of % positive cells of 3 different experiments.
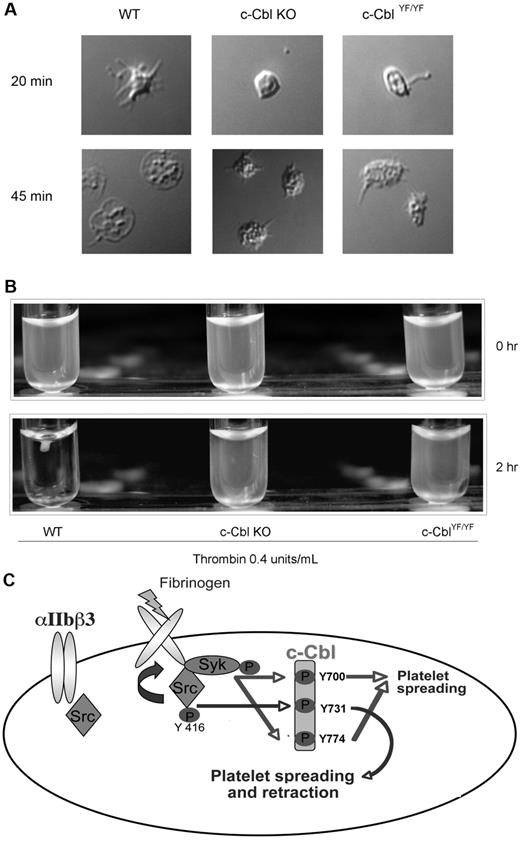
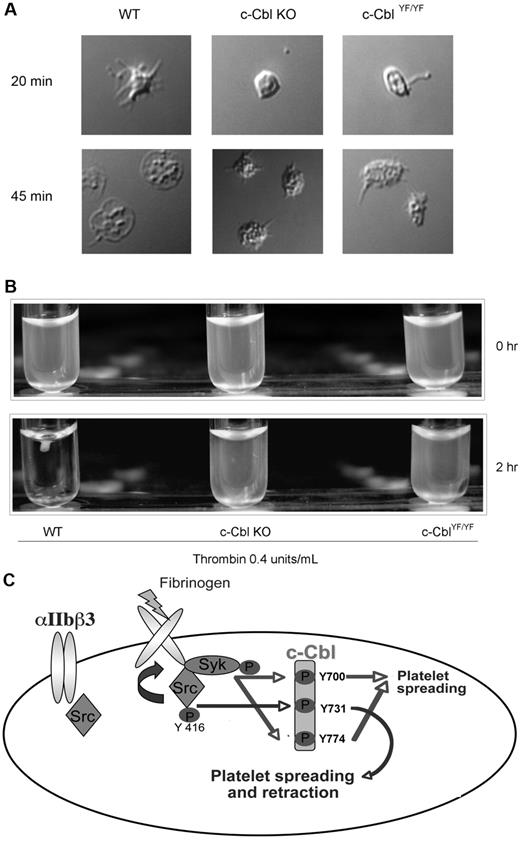
To further investigate the functional significance of c-Cbl tyrosine phosphorylation we evaluated the morphology of c-Cbl KO and c-CblYF/YF platelets with DIC imaging, wherein platelets membranes are intact. After initial contact with fibrinogen, adherent WT platelets exhibited actin-rich filopodial extensions and variable degrees of spreading after 45 minutes over fibrinogen, acquiring the characteristic shape of an almost completely spread platelet. In contrast, platelets from c-Cbl KO or c-CblYF/YF mice show a variable degree of spreading, being partly reduced with only few filopodial extensions that appear truncated or shorter than those in WT platelets. The formation of the truncated filopodia is SFK and Syk-dependent as inhibition of both the kinases totally abolished platelet-spreading (supplemental Figure 2A). The body of the platelet contains a variable number of actin nodules and even after 45 minutes of attachment over fibrinogen, mutant platelets failed to acquire the characteristic shape seen with WT platelets (Figure 7A).
Physiologic role of c-Cbl Y731 in platelet outside-in signaling. (A) Murine platelets were allowed to adhere to a fibrinogen-coated cover slip. After 3 times washing with PBS, adherent cells were fixed with 3.7% paraformaldehyde and analyzed with DIC imaging. (B) Clot retraction analysis of platelets from c-Cbl KO and c-CblYF/YF animals. (C) Model depicting the signaling events implicated in c-Cbl phosphorylation downstream of αIIbβ3: Fibrinogen binding to αIIbβ3 cause SFKs activation. The active kinases will phosphorylate c-Cbl on Y731 and activate Syk, which in turn, will phosphorylate c-Cbl Y700 and Y774. The phosphorylated tyrosines 700 and 774 will recruit SH2-domain containing proteins that play a role in platelet-spreading, while phosphorylated Y731 recruit molecules important for both platelet-spreading and retraction.
Physiologic role of c-Cbl Y731 in platelet outside-in signaling. (A) Murine platelets were allowed to adhere to a fibrinogen-coated cover slip. After 3 times washing with PBS, adherent cells were fixed with 3.7% paraformaldehyde and analyzed with DIC imaging. (B) Clot retraction analysis of platelets from c-Cbl KO and c-CblYF/YF animals. (C) Model depicting the signaling events implicated in c-Cbl phosphorylation downstream of αIIbβ3: Fibrinogen binding to αIIbβ3 cause SFKs activation. The active kinases will phosphorylate c-Cbl on Y731 and activate Syk, which in turn, will phosphorylate c-Cbl Y700 and Y774. The phosphorylated tyrosines 700 and 774 will recruit SH2-domain containing proteins that play a role in platelet-spreading, while phosphorylated Y731 recruit molecules important for both platelet-spreading and retraction.
In accordance to the defect seen on the actin cytoskeleton on spreading conditions, platelets from c-Cbl KO or c-CblYF/YF mice demonstrated a significant delay in clot retraction compared with WT platelets (Figure 7B). Even after addition of exogenous fibrinogen (0.1 mg/mL), mutant platelets failed to fully retract (supplemental Figure 2B). These results indicate the important role of c-Cbl and of the phosphorylated reside Y737 in cytoskeletal rearrangements.
Discussion
Platelet outside-in signaling is an important event in hemostasis and thrombosis as it mediates intracellular responses that regulate thrombus growth and stability. However, many of the signaling mechanisms downstream of integrin activation are poorly understood. c-Cbl has been identified as a protein activated by integrins8,19,20,25,26 and through its phosphorylated tyrosine residues, it mediates signaling cascades by recruiting Vav, PI3K, and Crk/CrkL.11,16,17 Nonetheless, the physiologic role of c-Cbl integrin-mediated signaling and the mechanism of phosphorylation of the tyrosine residues in platelets are not determined. We report that, on activation, c-Cbl undergoes tyrosine phosphorylation on its 3 major phosphorylation sites Y700, Y731, and Y774 and it plays a pivotal role in platelet αIIbβ3-mediated functional responses.
Using pharmacologic inhibitors we have identified that c-Cbl tyrosine residues are differentially phosphorylated in a SFK and Syk-dependent mechanism (outlined in Figure 7C). The role of SFKs in outside-in signaling has been established and is known to be proximal to the integrin.2,34 Of the several SFKs expressed in platelets, both Src and Fyn have been found to associate with the β3 integrin cytoplasmic tail.3,4,35 However, recent studies by Boylan et al9 have identified that FcγRIIA associates with αIIbβ3, which suggest that the integrin signals as a immune-like receptor. Hence Lyn kinase activation on integrin clustering might be also involved. At this point we have not identified the specific SFKs that are involved in the tyrosine phosphorylation of c-Cbl in platelets.
Syk activation is known to be dependent of SFKs activity both downstream of integrin activation2 and GPVI pathways,36 suggesting that SFKs and Syk are activated sequentially. The fact that c-Cbl Y731 is phosphorylated only by SFK (Figures 2,3B) suggests that this event is located proximal to integrin activation, rather than the phosphorylation of Y700 and Y774, which is dependent on Syk activity (Figure 3A). Because Syk catalytic activity requires SFK activation we would anticipate that phosphorylation of these tyrosine residues is temporally regulated.
Using over expression systems, Freshchenko et al12 demonstrated that the 3 tyrosine residues in c-Cbl are potentially phosphorylated by Src, Fyn, Yes, and Syk. The association between Src and c-Cbl has been shown to be mediated by Src-SH3 domain interaction with c-Cbl PXXP motifs37,38 ; the subsequent phosphorylation events and the preference of SFK for Y731 over Y700 and Y774 might relate to its recognition motif. Although the 3 tyrosines are surrounded by motifs recognized by Src, Y731 lies in a consensus sequence (Y731EAM) that contains elements considered highly specific for Src recognition.39,40
To understand the functional outcome of c-Cbl differential tyrosine phosphorylation we also examined the effect of inhibiting the kinases responsible for c-Cbl phosphorylation and correlated these results with 2 major outside-in signaling outcomes: platelet-spreading and clot retraction. We found excellent correlation between SFK inhibition (and hence inhibition of phosphorylation of all 3 tyrosines) and both spreading and clot retraction. However, Syk inhibition correlated with platelet-spreading but not with clot retraction. As Y731 phosphorylation was unaffected by Syk inhibition, we postulated that this phosphorylation plays an important role in platelet-spreading and clot retraction (outlined in Figure 7C). On the other hand phosphorylation of Y700 and Y774 might only play a role in spreading by the possible recruitment of Vav and Crk/CrKL. This is consistent with the decreased spreading phenotype observed in Vav1/3−/− platelets.41
To further evaluate the role of Y731 phosphorylation, we investigated platelet outside-in signaling in c-Cbl KO and in c-CblYF/YF. We chose to target Y731, in preference to Y700 or Y774, because this site is unique to c-Cbl, that is, it is not present in Cbl-b, and because several studies have showed that pY731 recruits p85 promoting the activation of PI3K.11,16,17 Therefore targeting this site in a mouse model would determine the in vivo significance of a c-Cbl–specific function that cannot be compensated for by Cbl-b. Relative to WT platelets, platelets from c-Cbl KO or c-CblYF/YF exhibited a partly reduced spreading when observed by confocal microscopy or with DIC imaging. We have eliminated the possibility that this defective spreading is because of deficiencies in integrin expression, activation, or granule secretion (Figure 6). Furthermore, analysis of the activation of the kinases involved in c-Cbl phosphorylation demonstrate that both SFK and Syk are equally activated in c-CblYF/YF and WT platelets (supplemental Figure 3B-C) which indicates that the defect seen is likely because of lack of Y737 phosphorylation alone. Another important observation is that the spreading defect of c-Cbl KO platelets is almost identical to platelets from c-CblYF/YF mice. Similarly, we observed a dramatic delay in the clot retraction of platelets from c-Cbl KO or c-CblYF/YF compared with those from WT mice. Even when we challenged the system by the addition of exogenous fibrinogen, the defect in clot retraction is present in c-Cbl KO and c-CblYF/YF platelets (supplemental Figure 2B). These results also indicate that c-Cbl E3 ubiquitin ligase activity is not playing a role downstream of integrin signaling as the RING finger domain is intact in c-CblYF/YF mice.42 Taken together our results demonstrate that c-Cbl's adapter function and more specifically phosphorylation of Y731 are of pivotal importance in platelet outside-in signaling.
An important consequence of c-Cbl Y731 phosphorylation in platelets is Syk-dependent phosphorylation of c-Cbl on Y700 and Y774. The levels of c-Cbl phosphorylation on residues equivalent to Y700 and Y774 are extremely low in mouse platelets allowed to spread on fibrinogen, probably because of the fact that murine platelets do not express FcγRIIA and thus do not spread well on fibrinogen. This limiting factor precluded us from analyzing the phosphorylation state of c-Cbl in WT versus c-CblYF/YF mouse platelets downstream of αIIbβ3 engagement. However, the observation that phosphorylation of Y700 and Y774 was delayed and reduced in c-CblYF/YF relative to WT mouse platelets after stimulation with the potent GPVI-specific agonist CRP, clearly demonstrates that phosphorylation of c-Cbl on Y731 is critical for phosphorylation of Y700 and Y774 in platelets (supplemental Figure 3D-E). This finding is consistent with results of previous studies in other cell types, which suggest the possibility that there is a positive cooperation between the tyrosine phosphorylation sites of c-Cbl.12,43
The mechanism responsible for the cooperative tyrosine phosphorylation of c-Cbl by SFKs and Syk is, however, not known. Previous studies have established that the interaction of Syk with c-Cbl involves the c-Cbl tyrosine kinase binding domain (TKB), which does not encompass Y731, and a phosphotyrosine residue at position 323 within Syk.44,45 Furthermore, we found that Syk is equally activated in c-CblYF/YF and WT platelets allowed to spread on fibrinogen (supplemental Figure 3C). These findings indicate that phosphorylation of c-Cbl Y731 is not involved in Syk activation per se. An alternative possibility is that phosphorylation of Y731 induces a conformational changes in c-Cbl that makes additional tyrosine residues more accessible to the kinase domain of Syk. Further studies are needed to determine the precise mechanism by which phosphorylation of c-Cbl on Y731 supports Syk-dependent phosphorylation of Y700 and Y774.
Considering that PI3K is by far the best known partner of Y731 and that PI3K function is of great importance in platelet outside-in signaling,46,47 phosphorylation of Y731 is an event that might be critical for proper localization and function of PI3K. Thus, we speculate that Y731 phosphorylation is rapidly needed after integrin engagement; being regulated by SFKs, whose activation occurs rapidly after fibrinogen binding, will promptly create a docking site for PI3K. Nonetheless, we cannot rule out the possibility that the defects seen in the c-CblYF/YF are because of some other unidentified Y731 partners. Consistent with our speculation, we have recently shown that PI3K activity is up-regulated in c-CblYF/YF osteoclasts, indicating that c-Cbl-PI3K interaction provides a regulatory effect in various signaling pathways.42 Whether this is the case in platelets remains to be investigated, but the similarities between integrin signaling in different cell types may indicate that the mechanism delineated here of αIIbβ3 signaling may be relevant to integrins in a variety of biologic contexts.
In conclusion, we propose a platelet outside-in signaling mechanism in which c-Cbl adapter function is necessary for the formation of signaling complexes in the appropriate location and time. As per our model (Figure 7C) interactions among c-Cbl, Src and Syk result in actin cytoskeleton rearrangement, filopodia and lamellipodia formation, spreading and clot retraction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Research Grants HL60683, HL81322, and HL 93 231 (S.P.K.), and AR055601 (A.S.) from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: L.B. designed and performed experiments, analyzed data, wrote the paper; W.L. provided important reagents; and A.S. and S.P.K. provided overall direction, designed experiments, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satya P. Kunapuli, Department of Physiology, Temple University School of Medicine, Philadelphia, PA; e-mail: spk@temple.edu; or Archana Sanjay, PhD, Department of Anatomy and Cell Biology, Temple University School of Medicine, Philadelphia, PA; e-mail: asanjay@temple.edu.
References
Author notes
A.S. and S.P.K. contributed equally and may be cited in either order.