Abstract
Abstract 1003
Patients with CD20+ Leukemia and Lymphoma (L/L) at diagnosis and a majority of those at relapse have a chemotherapy resistant phenotype and a dismal prognosis. Novel, non-chemotherapy-based therapies are desperately needed for this specific poor risk population. Natural Killer (NK) cells from cord blood (CB) play an important role in tumor surveillance post allogeneic stem cell transplantation (Beziat V et al, Leukemia, 2009) but cell number and tumor recognition limit adoptive NK cell therapy (Shereck/Cairo, PBC 2007). Genetically Engineered expanded CBNK cells with anti-CD19 chimeric receptor has been previously reported (Li L et al, Cancer Gene Ther. 2010).
We investigated the functional activities and cytolytic effect of chimeric anti-CD20 receptor (anti-CD20 CAR) engineered CBNK cells expanded with modified K562 (generously supplied by D. Camapana, MD, PhD) against CD20+ L/L both in vitro and in vivo.
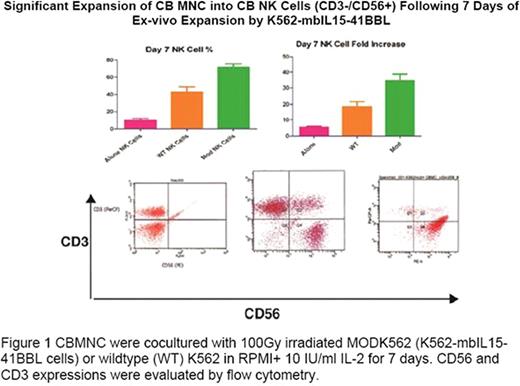
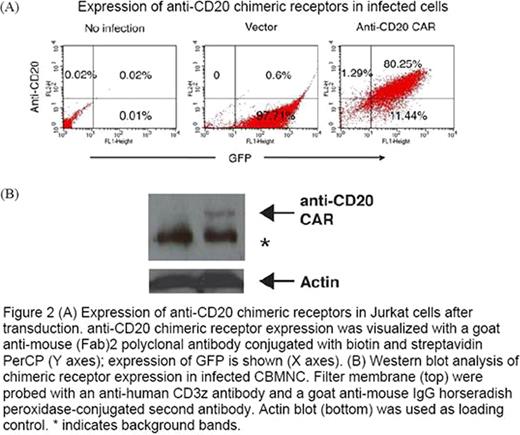
CBMNC were obtained by Ficoll-Hypaque density gradient centrifugation. CBMNC were cocultured with 100Gy irradiated MODK562 (K562-mbIL15-41BBL cells) or wildtype (WT) K562 in RPMI+ 10 IU/ml IL-2 for 7 days. Medium was changed every 2 days with fresh medium and IL-2. CD56 and CD3 expression were evaluated by flow cytometry. NK activation marker LAMP-1 and cytolytic mechanism markers, perforin and granzyme B were evaluated by flow cytometry in expanded CBNK. NK cytotoxicity was assessed by europium release assay at 20:1 E:T ratio against CD20+ Ramos. Luciferase expressing (ffLuc+) Ramos were generated by transfecting ffLUCZeo-pcDNA plasmid DNA (generously supplied by L. Cooper, MD, PhD) and selected by Zeocin. In vivo ffLuc+ Ramos engraftment in NOD/SCID mice was verified using a Xenogen IVIS-200 system (Caliper Life Sciences). 6 weeks old xenografted mice were treated for 5 weeks with PBS, Ramos, 5×106 WTK562 expanded (E) CBMNC, 5×106 or 5×107 MODK562E CBMNC IP. Tumor growth was monitored by volume using bioluminescent imaging and mice survival was observed for 10 weeks. Different retrovirus preps that express the anti-CD20 CAR or control GFP only (CAR−) were generated independently. The anti-CD20 CAR was constructed in a MSCV-anti-CD20BB-CD3-zeta-GFP plasmid (generously supplied by Dario Campana, MD, PhD, St. Jude Children's Hospital, Memphis, Tenn). MSCV-anti-CD20BB-CD3-zeta-GFP or control empty vector (MSCV-GFP) were transfected to 293T cells with helper plasmids pEQ-PAM3(-E) and pRDF. Conditioned medium containing retrovirus was harvested 48 and 72 h after transfection. Jurkat cells were used to examine infection efficiency. Anti-CD20 CAR expression was examined by flow cytometry with staining cells with goat anti-mouse (Fab)2 polyclonal antibody conjugated with biotin (Jackson Immunoresearch, West Grove, PA, USA) followed by PE-conjugated streptavidin and the expression was further confirmed by western blot of whole cell lysates for CD3zeta.
CBNK cells were significantly increased compared to WTK562 and media alone (72 ± 4 {35-fold} vs 43 ± 6 vs 9 ± 2%, p<0.01) (Figure 1). Cytotoxicity against Ramos was increased compared to WTK562 CBMNC (p<0.01); along with increased expression of NK activation marker, CD107a (p<0.05), perforin and granzymeB (42 ± 1.5 vs 15 ± 0.5%,p<0.001; 22 ± 0.5 vs 11 ± 0.3%, p<0.0.001, respectively). At 5 weeks, tumor volume in mice receiving either dose of MODK562E CBMNC was significantly decreased vs WTK562E CBMNC (2 ± 0.6 and 0.4 ± .05 vs 3 ± 0.25/mm3, p=0.0086, p=0.001, respectively and luminescence p<0.01). Xenografted mice receiving MODK562E CBMNC showed increased survival vs WTK562E (p<0.001). CAR+ and CAR− retrovirus supernants infected Jurkat cells efficiently. 3 independent retrovirus preps infected Jurkat cells with 97.71% CAR−GFP+ vs 80.25% CAR+ GFP+, 40.25% CAR−GFP+ vs 74.03% CAR+ GFP+, and 88.57% CAR−GFP+ vs 62.64% CAR+ GFP+. The anti-CD20 CAR expression was further confirmed by western blots (Figure 2).
CBMNC stimulated with MODK562 was associated with significantly increased NK LAMP-1, perforin and granzyme B expression, enhanced cytotoxicity in-vitro and in-vivo survival. Anti-CD20 CAR expressed was confirmed in infected cells. Future directions include infecting MODK562 expanded CBNK with retrovirus expressing anti-CD20 CAR and examining the cytotoxicity activity of engineered CBNK against L/L in-vitro and survival in xenogafted mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.