Abstract
Thrombocytopenia(TP) is common in patients with Human Immunodeficiency Virus (HIV) infection, reported in as many as 10–30% of patients and seen more often in IV drug users and advanced HIV infection even in post-HAART era. Both decreased production of platelets and increased destruction have been proposed as mechanisms of thrombocytopenia and at least 2 different clinical scenarios have been described: patients with very advanced AIDS, being thrombocytopenia another manifestation of bone marrow suppression; and patients without significant T-cell depletion in whom thrombocytopenia develops likely as an immune phenomenom, “ITP-like” HIV-related thrombocytopenia (HIV-TP).
In HIV-TP, common treatments for classical ITP have been reported including corticosteroids, danazol, splenectomy, Intravenous Gammaglobulin, interferon, Anti-D, dapsone and splenic radiation with varying degrees of success with the highest responses seen with the use of HAART.
Romiplostim (RMP) and Eltrombopag (ETP) are thrombopoeitin agonists (TA) approved in 2009 for refractory ITP and recently proposed to be used as first-line agents. No reports of the use of TA in patients with HIV-ITP are available to date.
5 patients with HIV-related ITP were treated with TP in our centers between 2009 to 2011. Mean age was 48 years (25–61), 4 males and 1 female. All of the patients had failed or relapsed after prednisone, 3 of the patients had also failed multiple treatments including splenectomy (1 patient), IVIG, Rho(D) immune globulin, Rituximab, danazole and vincristine. All but 1 of the patients were on HAART by the time of treatment and CD4 counts were <200 cells/μL in 2 patients (10–157 cells/ μL).
All 5 patients were treated initially with RMP at the starting dose of 1 mcg/Kg and titrated as per guidelines. One patient requested to be switched to ETP and developed an acute lower extremity DVT and symptomatic PE 1 week after starting the medication.
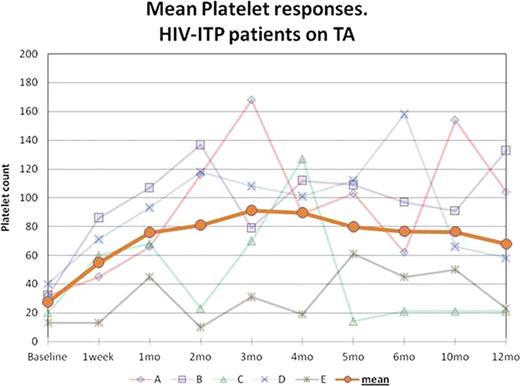
Responses to treatment are shown in graph 1. Mean baseline Platelet count was 27 × 103/μL (13–40 × 103/μL) and it increased to 66 × 103/μL (45–86 × 103/μL) within 1 week and 76 × 103/μL (45–107 × 103/μL) after 1 month of therapy, 2.8 times the baseline (2.1 – 3.4 × baseline value). 4 patients achieved a platelet count ≥50 × 103/μL within 1 month. 4 of the patients had durable responses for ≥ 12 months not requiring dose adjustments or modifications; the other patient had several compliance issues and missed several doses of RMP, his platelet count improved every time the medication was restarted.
Byrnes:Amgen: Honoraria, Speakers Bureau.
This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.