Abstract
Abstract 1323
The importance of the tumor microenvironment for cancer development, progression and resistance to treatment has recently been recognized. Our group was first to report the contribution of bone marrow (BM) derived mesenchymal stromal cells (MSCs) for tumor development and metastasis. BM is also the dynamic microenvironment (niche) for normal and malignant hematopoietic stem cells (HSC) with high local concentrations of growth factors, chemokines and cytokines. The maintenance of HSCs quiescence and normal hematopoiesis require complex bidirectional interactions between the BM niches and HSCs. Accumulating evidence has shown that the BM microenvironment also plays a pivotal role in the pathophysiology and propagation of leukemia. Leukemia cells undergo spontaneous apoptosis once they are removed from the in vivo microenvironment and placed in suspension cultures without supportive stroma. The understanding of the interactions between leukemic cells and their BM niche is also critically important for leukemia therapy. We here describe a novel artificial bone and bone marrow model mimicking the human hematopoietic microenvironment by using human BM derived MSCs and endothelial colony-forming cells (ECFCs). MSCs and ECFCs were isolated from heparinized human bone marrow or peripheral blood through an initial adhesion step, grown in specific media and then subcutaneously injected into the flanks of the NOD/SCID/IL-2r-gammanull mice, where they developed into bone-like tissues with high osteoblast activity after 10 weeks (Figure 1). Histochemical stains confirmed the bone structures and also showed that these artificial bones contained typical bone marrow cavities constituting a robust hematopoietic environment. In vivo imaging with Osteosense confirmed the presence of hydroxylapatite, and luciferase imaging of firefly luciferase labeled human leukemic cells demonstrated the engraftment of MOLM13/Luc/GFP leukemic cells in the extramedullary BM sites. The extramedullary BM was markedly hypoxic, as shown by Pimonidazole staining, another critical feature of the BM microenvironment. Factors critical for MSC to support the normal and leukemic hematopoiesis are largely unknown and cannot be studied since human MSC do not engraft reliably in xenograft models. We therefore investigated the possibility of genetically modifying MSC in this system and found a significant reduction (50 ± 6%, p<0.001) in MOLM13 cell engraftment in extramedullary BM generated with HIF1-alpha knockdown MSCs (1449 ± 194 cells/mm2), compared to vector controls (3037 ± 496 cells/mm2). This finding indicates that the HIF1-alpha expression in stromal cells is a critical component for the engraftment of leukemic cells in the physiologically hypoxic BM microenvironment. These results, for the first time, establish an in vivo bone and bone marrow model with a genetically controlled human microenvironment. Close modal
Figure 1
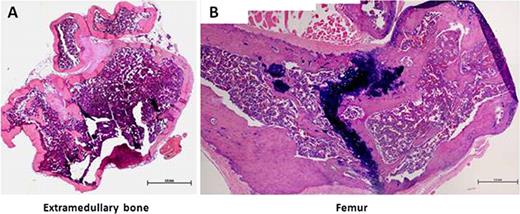
Establishment a human bone marrow microenvironment in NOD/SCID/IL-2r-gammanullmice. Representative hematoxylin and eosin (H&E) staining (shown at low magnification) shows an overview of the extramedullary bones with the typical bone structures. Scale bar: 1 mm.
Figure 1
Establishment a human bone marrow microenvironment in NOD/SCID/IL-2r-gammanullmice. Representative hematoxylin and eosin (H&E) staining (shown at low magnification) shows an overview of the extramedullary bones with the typical bone structures. Scale bar: 1 mm.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011