Abstract
Abstract 1534
There are no data on the role of post-consolidation therapy with gemtuzumab ozogamicin, (GO, Mylotarg) in children with AML. The NOPHO-AML 2004 protocol included two induction courses and four consolidation courses followed by a post-consolidation randomization to GO or no further therapy (ClinicalTrials.gov identifier NCT00476541).
GO was administered as 5 mg/m2 four weeks after last consolidation and repeated once after three weeks. We randomized 120 patients; 59 to receive GO, 61 to no further therapy. The children in the two groups had similar distribution of sex, age, WBC, and cytogenetics. Treatment was given as randomized in 113 (94% of the patients). Survival was analyzed on an intention to treat basis. The median follow-up for patients alive was 3.2 years (range 0.9 – 7.0).
Toxicity data from the children who received GO showed modest elevation of transaminase and bilirubin but without signs of VOD. No significant decrease in hemoglobin was observed whereas severe neutropenia occurred in 96% of the patients. Recovery to neutrophils > 0.5 lasted a median of 15 days. Febrile neutropenia followed 39% of the GO courses but none were life-threatening. A moderate decline in platelet count was noted with platelets < 50 × 109/L in 27% and transfusion was given following 11% of the GO courses.
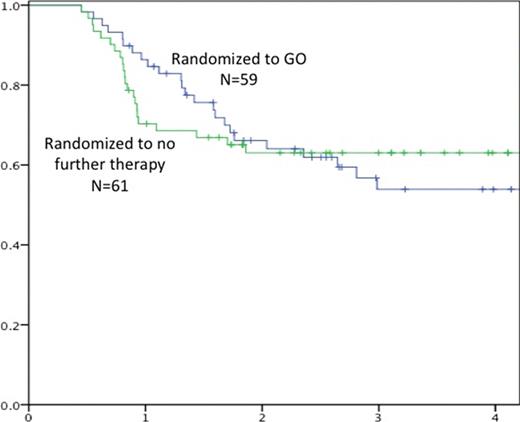
Relapse occurred in 22 and 23 of those randomized to GO or no further therapy, respectively. The median time to relapse was 16 months in GO treated vs. 10 months in those receiving no GO (n.s.) (figure). Therapy-related MDS developed in two patients treated with GO. The 3-year EFS and OS in those randomized to receive GO vs. no further therapy was 54% vs. 63% (n.s.) and 75% vs. 84% (n.s.), respectively. The results were similar in all subgroups analyzed.
In conclusion GO therapy post consolidation was well tolerated with severe neutropenia, modest thrombocytopenia and no clinical liver toxicity. GO did not change the frequency of relapses but there was a non-significant delay in the time to relapse from 10 to 16 months for patients randomized to GO.
Hasle:Pfizer: Research Funding. Off Label Use: A randomized study of gemtuzumab ozagamicin in childhood AML is presented.
Author notes
Asterisk with author names denotes non-ASH members.