Abstract
Abstract 1793
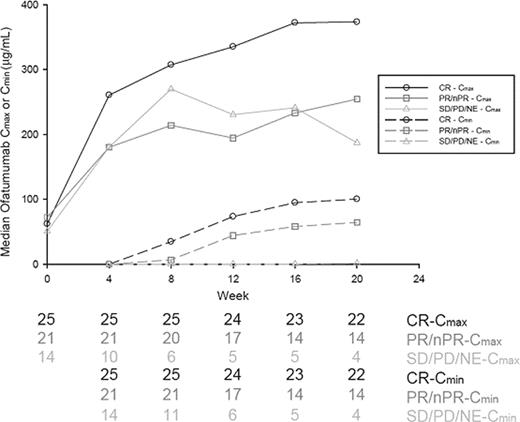
Results: Seven pts (4 male) with a medianLittle is known about the pharmacokinetics (PK) and pharmacodynamics of CD20 monoclonal antibody (mAb) with chemotherapy in patients (pts) with CLL. Ofatumumab (O) is a human mAb targeting a membrane-proximal small-loop epitope on CD20 and mediates efficient complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. Safety and efficacy of O at 2 dose levels in combination with fludarabine and cyclophosphamide (FC) were evaluated in previously untreated pts with CLL. Relationship between O PK, baseline characteristics, and clinical outcomes were studied.
Pts with active CLL were randomized to O 500 mg (n=31) or 1000 mg (n=30) on Day 1 with F 25 mg/m2 and C 250 mg/m2 on Days 2–4 (Course 1) or Days 1–3 (Courses 2–6) every 4 weeks for 6 courses. O dose at Course 1 was 300 mg for both groups. Response (1996 NCI-WG criteria) was assessed by an Independent Review Committee up to 3 months after last course. Serial blood samples were collected at Courses 1 and 6 for noncompartmental PK analyses; pre- and post-infusion samples were collected at other courses. Relationship between PK parameters and baseline pt characteristics and disease factors was evaluated by univariable and multivariable analyses. Associations between PK and complete response (CR), overall response (OR), or progression-free survival (PFS) were explored using univariable regression analyses.
22/31 (71%; 500 mg) and 19/30 (63%; 1000 mg) of pts received all 6 O doses; 2 pts at 1000 mg did not receive FC for Course 6. CR (primary endpoint) rates of 32% and 50% and OR rates of 77% and 73% were observed in the 500 mg and 1000 mg groups, respectively. O PK parameters are summarized (Table). O PK at Dose 6 appeared proportional to dose. Factors associated with PK in multivariable analyses are shown (Table). The factor most associated with PK at Dose 1 was sex, with higher Cmax/AUC and lower CL and Vss/longer t½ in women vs. men. PK at Dose 6 was not consistently associated with any factor tested.
Median Cmax and Cmin values were similar at first dose between pts who had CR, partial response (PR)/nPR, and stable disease (SD)/progressive disease (PD; Figure); at later doses, median Cmax and Cmin values appeared different between CR and the other groups, although number of subjects decreased over time, especially in the SD/PD group. Based on univariable analyses, higher Cmax and Cmin at Dose 3 and higher Cmax and AUC at Dose 6 were associated with increased likelihood of CR (P<.05); higher Cmin before Dose 6 was associated with increased likelihood of OR and longer PFS.
PK of O in combination with FC appeared proportional to dose after repeated dosing. Higher concentrations at Doses 3 and 6 were associated with CR. O PK was similar at first dose and different at later doses between patients who had CR, PR/nPR, and SD/PD, suggesting that response to O-FC treatment leads to clearance differences due to decreased B-cell mass and thus concentration differences with continued dosing. Further analyses of associations between disease-related factors, PK, and treatment response will be performed at study completion.
| Dose 1 . | PK Parametersa . | Factors Associated with PK Parametersb . | |||
|---|---|---|---|---|---|
| . | n . | 300 mg . | Variable . | Estimatec . | P value . |
| Cmax (mg/mL) | 60 | 62.3 (48) | 0.766 | <.001 | |
| Sex (F/M) Hemoglobin | 0.103 | <.001 | |||
| Cmind (mg/mL) | 57 | 0.5 (172) | Sex (F/M) | 0.524 | .006 |
| % BM involvement | –0.040 | <.001 | |||
| AUC (mgh/mL) | 50 | 2154 (100) | Sex (F/M) | 0.924 | <.001 |
| CL (mL/h) | 50 | 139 (100) | Sex (F/M) | –0.924 | <.001 |
| Vss(L) | 50 | 4.23 (34) | Sex (F/M) | –0.555 | <.001 |
| Hemoglobin | –0.070 | .002 | |||
| t½(days) | 50 | 0.796 (86) | Sex (F/M) | 0.739 | .001 |
| % BM involvement | –0.030 | .003 | |||
| Dose 1 . | PK Parametersa . | Factors Associated with PK Parametersb . | |||
|---|---|---|---|---|---|
| . | n . | 300 mg . | Variable . | Estimatec . | P value . |
| Cmax (mg/mL) | 60 | 62.3 (48) | 0.766 | <.001 | |
| Sex (F/M) Hemoglobin | 0.103 | <.001 | |||
| Cmind (mg/mL) | 57 | 0.5 (172) | Sex (F/M) | 0.524 | .006 |
| % BM involvement | –0.040 | <.001 | |||
| AUC (mgh/mL) | 50 | 2154 (100) | Sex (F/M) | 0.924 | <.001 |
| CL (mL/h) | 50 | 139 (100) | Sex (F/M) | –0.924 | <.001 |
| Vss(L) | 50 | 4.23 (34) | Sex (F/M) | –0.555 | <.001 |
| Hemoglobin | –0.070 | .002 | |||
| t½(days) | 50 | 0.796 (86) | Sex (F/M) | 0.739 | .001 |
| % BM involvement | –0.030 | .003 | |||
| Dose 6 . | PK Parametersa . | Factors Associated with PK Parametersb . | |||||
|---|---|---|---|---|---|---|---|
| . | n . | 500 mg . | n . | 1000 mg . | Variable . | Estimatec . | P value . |
| Cmax (mg/mL) | 22 | 201 (30) | 18 | 426 (35) | b2-microglobulin | –0.000 | .012 |
| BSA | –0.889 | .001 | |||||
| Cmind (mg/mL) | 22 | 34 (313) | 18 | 60 (1179) | CD5+CD19+ at Dose 5 | –0.003 | .001 |
| Bulky lymph nodes | –1.550 | .034 | |||||
| AUC (mgh/mL) | 16 | 145,453 (54) | 16 | 377,021 (37) | 17p del | –0.623 | .009 |
| Dose | 0.001 | <.001 | |||||
| CL (mL/h) | 17 | 6.4 (38) | 16 | 5.5 (25) | # of nodal sites | –0.041 | .027 |
| BSA | 0.714 | .019 | |||||
| Vss(L) | 16 | 5.17 (27) | 16 | 5.34 (47) | 17p del | –0.749 | .004 |
| t½(days) | 16 | 23.2 (31) | 16 | 28.6 (43) | CD5+CD19+ at Dose 5 | –0.000 | .001 |
| Dose | 0.001 | .017 | |||||
| Dose 6 . | PK Parametersa . | Factors Associated with PK Parametersb . | |||||
|---|---|---|---|---|---|---|---|
| . | n . | 500 mg . | n . | 1000 mg . | Variable . | Estimatec . | P value . |
| Cmax (mg/mL) | 22 | 201 (30) | 18 | 426 (35) | b2-microglobulin | –0.000 | .012 |
| BSA | –0.889 | .001 | |||||
| Cmind (mg/mL) | 22 | 34 (313) | 18 | 60 (1179) | CD5+CD19+ at Dose 5 | –0.003 | .001 |
| Bulky lymph nodes | –1.550 | .034 | |||||
| AUC (mgh/mL) | 16 | 145,453 (54) | 16 | 377,021 (37) | 17p del | –0.623 | .009 |
| Dose | 0.001 | <.001 | |||||
| CL (mL/h) | 17 | 6.4 (38) | 16 | 5.5 (25) | # of nodal sites | –0.041 | .027 |
| BSA | 0.714 | .019 | |||||
| Vss(L) | 16 | 5.17 (27) | 16 | 5.34 (47) | 17p del | –0.749 | .004 |
| t½(days) | 16 | 23.2 (31) | 16 | 28.6 (43) | CD5+CD19+ at Dose 5 | –0.000 | .001 |
| Dose | 0.001 | .017 | |||||
Geometric mean (% coefficient of variation)
Results of backward elimination from full model
Estimate >0 associated with positive influence; estimate <0 associated with negative influence
Cmin after first infusion or before sixth infusion
BM, bone marrow; BSA, body surface area
Wierda:GlaxoSmithKline: Research Funding; Genentech: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Merck: Consultancy; Abbott: Research Funding. Off Label Use: Ofatumumab is an anti-CD20 monoclonal antibody approved for the treatment of fludarabine- and alemtuzumab-refractory chronic lymphocytic leukemia, and is currently under development for the treatment of B-cell malignancies (chronic lymphocytic leukemia, diffuse large B-cell lymphoma, Waldenstroms macroglobulinemia and follicular lymphoma), as well as autoimmune diseases (rheumatoid arthritis and multiple sclerosis). Jewell:GlaxoSmithKline: Employment. Kipps:Gilead Sciences: Consultancy, Research Funding; GSK: Research Funding; Genentech: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Abbot Industries: Research Funding; Celgene: Consultancy, Research Funding; Igenica: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Dürig:Santaris Pharma: Consultancy, Research Funding; GSK: Speakers Bureau; Roche: Speakers Bureau; Celgene: Research Funding. Stilgenbauer:Genmab: Research Funding; GSK: Consultancy, Honoraria, Research Funding. Smolej:Roche: Honoraria, Travel Grants; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Travel Grants; Genzyme: Honoraria, Travel Grants. Hernandez-Ilizaliturri:Genmab: Research Funding; Celgene: Honoraria; Amgen: Research Funding. Fang:PharStat: Employment; GSK: Consultancy; Gilead Sciences: Consultancy; Pharmasset Inc: Consultancy. Gorczyca:GlaxoSmithKline: Employment. Chan:GlaxoSmithKline: Employment. Gupta:GlaxoSmithKline: Employment. Lisby:Genmab A/S: Employment.
Author notes
Asterisk with author names denotes non-ASH members.