Abstract
Abstract 1862
Multiple Myeloma (MM) and its treatment are associated with impaired humoral and cellular immunity (CI). Lenalidomide (Len) is thought to mediate its anti-MM effect in part via stimulation of in vivo T cell, NK cell and NKT cell activity. However, as we have previously demonstrated (Hsu et al, Blood, 2011;117:1605), the immunostimulatory effects of Len are substantially abrogated by co-administration of high doses of Dexamethasone (Dex). It is unknown whether the efficacy of the Len/Dex induction regimen is maintained in newly diagnosed MM if the dose of Dex is lowered to preserve CI. In a prospective study (PMCC HREC #05/56), we examined both anti-MM responses and CI using a regimen of low dose Len and Dex induction followed by consolidation with autologous stem cell transplant (ASCT) in previously untreated patients with MM.
Twenty patients were enrolled. Induction therapy was with four 28 day (d) cycles of Len 15 mg d1 − 21 and Dex 20 mg weekly followed by hematopoietic stem cell (HSC) mobilization with cyclophosphamide 2 – 4 gm/m2 and G-CSF 10mcg/kg/day, and a melphalan 140 – 200mg/m2 conditioned ASCT. Maintenance with Rev 25mg d1-21 of a 28d cycle was commenced on d21-35 post ASCT until progression. Interim assessments of response were made post-induction, post-ASCT and on 31/05/2011 using IMWG uniform response criteria. Assessments of CI were undertaken at enrolment and again at the end of 4 cycles of induction by flow cytometric analysis of peripheral blood lymphocyte subsets (CD19+, CD3+, CD4+, CD8+, CD4+/CD25+/CD127+ Treg, CD3−/ CD16+/CD56+ NK cells), T cell proliferation to allogeneic stimulators (ratio 1:1) in a 7d mixed lymphocyte reaction (MLR) and analysis of NK function by cytotoxicity against K562 targets. All data were compared to age-matched controls, and analysed for statistical significance using a student T-test.
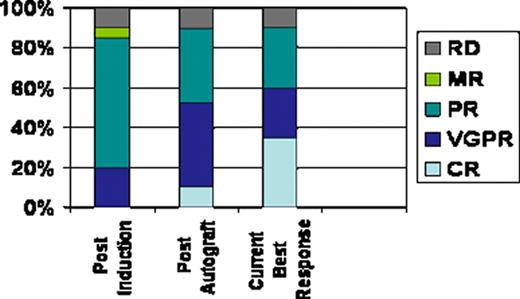
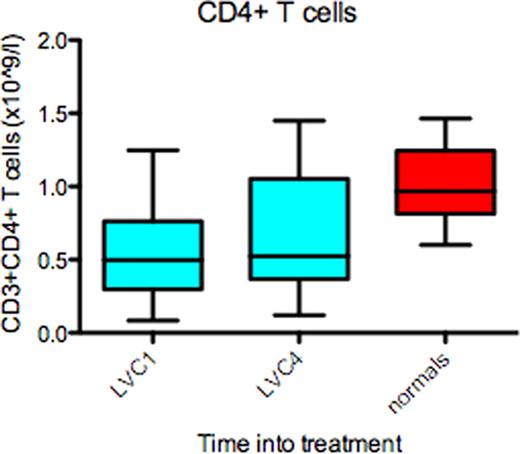
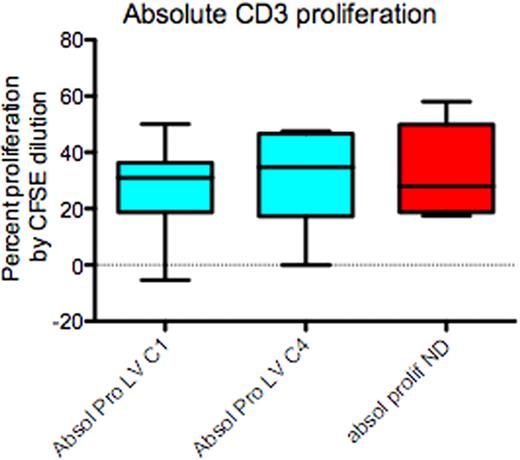
All patients have completed induction and ASCT. Median age=57.5 yrs, (range 44 – 70); male= 15; IgG = 10, IgA = 6 and light chain = 4. Stage at enrolment ISS1 = 9, ISS2 = 9 and ISS3 = 2. Median harvested CD34+ cells=10.8×106 /kg (range 4.9 – 40.6). The median number of CD34+ cells infused = 4.8×106 / kg (range 2.9 – 11.5) and median time to recovery of neutrophils > 0.5 ×109/L and platelets > 20 ×109/L was 12d (range 9 – 18) and 11d (range 8 – 25) respectively. The post-induction, overall response rate was 85%, very good partial response (VGPR)=20%, partial response (PR)= 65%, stable disease =5%, and 10% were refractory. Post-autograft responses improved to complete response (CR)=10%, VGPR= 40% and PR=35%. At a median follow-up of 17 months (range 5 – 29 months), the best response achieved to date is 35% CR, 25% VGPR and 30% PR (Fig 1). Immunology: CI data is available on 19 patients. Compared-to age matched controls, patients at enrolment had reduced CD3+ (p≤0.05), CD4+ (p≤0.01) (Fig 2)and Treg (P≤0.01) numbers and reduced NK cell function (p<0.05); however CD8+ T cell, NK cell and B cell numbers were normal. Following four cycles of Len/Dex therapy, CD3+, CD4+ and Treg numbers all increased, but remained below those of normal controls. Conversely, circulating B lymphocyte numbers fell substantially (P≤0.01) and NK cell function remained significantly impaired (p<0.05) following induction therapy. However, functional analysis of CD3+ (Fig 3), CD4+ and CD8+ proliferation in an MLR at enrolment and following induction therapy was identical to that of controls.
This novel treatment regimen of low dose Len and low dose Dex followed by ASCT in untreated patients with MM is associated with high response rates and successful HSC collection. The depth of response progressively improved following ASCT. Cellular immunology in MM patients at diagnosis shows specific reductions in CD4+ and Treg cell numbers, and NK cell function that is not rescued by Len therapy when co-administered with Dex, even at low doses, which induced a further reduction in CD19+ cells. However despite these deficiencies, preserved proliferative capacity of both CD4+ and CD8+ T-cells is seen at diagnosis and following this induction therapy, suggesting that with the low dose Len-Dex regimen, the immune environment is conducive to novel strategies that are aimed at inducing adaptive anti-MM immune responses.
Harrison:Celgene: Honoraria, Research Funding. Off Label Use: Low dose lenalidomide in newly diagnosed myeloma. Neeson:Celgene: Research Funding. Prince:Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.