Abstract
Abstract 1879
Multiple myeloma (MM) can be cured by allogeneic stem cell transplantation (SCT), which induces PCR-negative molecular remission (MR) in patients with MM. Autologous peripheral blood stem cell transplantation (ASCT) followed by maintenance therapy can also induce PCR-negative MR in MM patients, with a progression-free survival (PFS) rate of 100% at 42 months (JCO 2010). Accordingly, it is essential to assess the depth of remission using clonotype-specific PCR primers in the treatment of MM. To prepare primers for amplification of rearranged regions of the IgH gene specific to individual MM patients, fresh or frozen bone marrow (BM) or peripheral blood cells containing MM cells are required. However, these materials are not always available, particularly when patients are diagnosed and treated at local hospitals. Archival BM slides that had been prepared for diagnosis may serve as a source of DNA for PCR-based minimal residual disease (MRD) detection. Therefore, we examined whether DNA extracted from archival BM slides was useful for designing clonotype-specific IgH PCR primers for MRD detection in autografts as well as in the BM in the post-ASCT setting.
Twenty-two Japanese patients (9 men and 13 women; median age, 56 years; age range, 48–68) with newly diagnosed MM who received various induction regimens prior to ASCT were retrospectively analyzed. All patients had achieved a very good partial response (VGPR) or complete response (CR) after ASCT. BM slides from 19 MM patients, which had been stored in a steel box at room temperature for 19 to 90 months (median, 60 months), and fresh BM cells from 3 MM patients, obtained at first diagnosis, were subjected to DNA extraction using the QIAamp DNA Micro Kit (QIAGEN). The IGH Gene Clonality Assay Kit (InVivoScribe Technologies) was used to detect IgH gene rearrangements in the extracted DNA. The CDR III region of the IgH gene was sequenced to design clonotype-specific primers for amplification of the rearranged sequence in MM patients. BM slides prepared at 3 to 12 months post-ASCT were also subjected to DNA extraction, followed by MRD detection using clonotype-specific PCR primers.
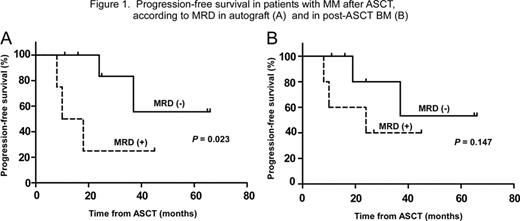
IgH gene clonality was detected in 6 of 8 (75%) unstained slides and 4 of 5 (80%) Giemsa-stained slides that had been preserved for less than 5 years. For slides that had been preserved for more than 5 years, IgH gene clonality was detected in 5 of 11 (45%) unstained slides and 1 of 4 (25%) Giemsa-stained slides. Clonotype-specific IgH PCR primers were prepared in 9 of 19 cases (47%) using BM slides and in 3 of 3 cases (100%) using fresh BM cells. DNA in peripheral blood stem cell autografts of 12 patients who had achieved a VGPR or CR after ASCT was subjected to PCR to amplify clonotype-specific rearranged IgH gene sequences; rearranged sequences were amplified in four patients. Within 2 years post-ASCT, 3 of 4 (75%) patients with MRD-positive autografts (Group A) developed progressive disease, whereas all of the 8 patients with MRD-negative autografts (Group B) remained in remission. Group A showed a higher risk of progression than Group B (P = 0.023) (Figure 1A), although overall survival was comparable between the two groups (P =0.371). When BM slides (10 patients) and fresh BM cells (2 patients) obtained after ASCT were examined for the presence of MRD using clonotype-specific PCR primers, the rearranged IgH fragments were amplified in 5 of 12 patients. PFS of 5 MRD-positive patients tended to be shorter than that of 7 MRD-negative patients (P = 0.147) (Figure 1B).
Archival BM slides can be used as a source of DNA to prepare clonotype-specific primers for MRD detection in MM patients whose cyropreserved myeloma cells are not available for DNA preparation. Furthermore, MRD status determined by PCR-based clonality analysis was useful in predicting prognosis of patients with MM. This approach enables us to select appropriate consolidation/maintenance therapy in MM patients based on their MRD status. A large multi-institutional study is currently underway to verify the clinical relevance of MRD detection using this technique.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.