Abstract
Abstract 2022
HDT/ASCT is the preferred treatment for relapsed and refractory HL patients (pts) with chemosensitive disease, with cure rates approximating 40–60%. The role for HDT/ASCT in chemoresistant HL is less well defined and many centers do not offer this treatment to such patients. Since 1985, HDT/ASCT has been recommended in British Columbia (BC) for all HL pts with progressive disease despite primary ABVD-type therapy, irrespective of response to salvage therapy. We sought to evaluate the long-term outcomes of HL pts whose disease was resistant to chemotherapy preceding HDT/ASCT.
We reviewed all HL pts who underwent HDT/ASCT for primary progression (PP) or first relapse (1REL) after initial treatment with chemotherapy +/− radiation. Primary progression (PP) was defined as progression during or within 3 months of completion of initial therapy. Pts were considered to have: chemoresistant (R) disease = stable disease or progression on chemotherapy preceding HDT/ASCT; chemosensitive (S) disease = clinical and/or radiographic response to chemotherapy preceding HDT/ASCT; or untested (U) if no salvage chemotherapy was given. Clinical and laboratory data were obtained from the BC Cancer Agency Lymphoid Cancer Database, the Leukemia/BMT Program of BC Database and from hospital, clinic, and physician records.
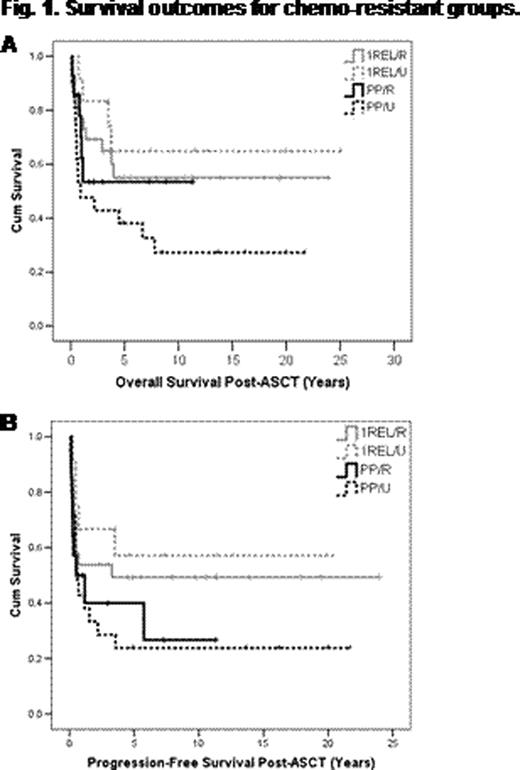
251 pts underwent HDT/ASCT for PP (n=90 36%) or 1REL (n=161 64%) between 1985–2011: male 53%; median age at diagnosis 28 y (range 16–59 y), at HDT/ASCT 31 y (range 31–62 y). Characteristics at diagnosis were: advanced stage(stage IIB, II bulky, III or IV) 94%; stage 3–4 60%; B symptoms 57%; bulk (≥10 cm) 42%; primary therapy: ABVD/ABVD-like, 95%; MOPP-like 5%; combined modality therapy 31%. Salvage therapy prior to HDT/ASCT included MVPP (28%); COP/COPP (22%); GDP (27%); no chemotherapy (13%); other (11%). RT was given with salvage therapy in 19%: alone, 27%; with chemotherapy, 73%. Conditioning regimen was with CBV/CBVP in the majority of cases (88%); BEAM (11%); other (1%). At a median follow-up for living pts of 8 y (range 0.2 – 25 y), 136 pts (54%) were alive free of HL; 89 pts (35%) have relapsed. For all pts, median overall (OS) and progression free survivals (PFS) were 21.7 y (95% CI 16.0–27.5) and 17.3 y (95% CI 9.8–24.8), respectively. 13 pts (5%) died of complications related to or within 1 month of HDT/ASCT, 6 (2%) from secondary malignancies, 7 (3%) from unrelated causes. 199 pts (56 PP, 143 1REL) had information available regarding response to salvage therapy. Of the 56 PP pts, 14 (25%) had chemoresistant disease (PP/R); 21 (38%) did not receive salvage therapy and thus were untested (PP/U); 21 (38%) had chemosensitive disease (PP/S). 10-y PFS for PP/R, PP/U, and PP/S groups were 27%, 24%, and 40%, respectively; 10-y OS were 53%, 27%, and 53%, respectively. Of the 143 1REL pts, 26 (18%) had chemoresistant disease (1REL/R); 12 (8%) did not receive salvage therapy (1REL/U); 105 (73%) had chemosensitive disease (1REL/S). 10-y PFS for 1REL/R, 1REL/U, and 1REL/S groups were 49%, 57%, and 58%, respectively; 10-y OS were 55%, 65%, 69%, respectively. OS and PFS for the chemoresistant groups (PP/R, PP/U, 1REL/R) and 1REL/U are shown in Figures 1A and 1B respectively. To evaluate impact of chemoresistance on outcomes, PP/R (pts resistant to both primary and salvage therapy, “double-resistant”) and PP/U pts (resistant to primary therapy, “single-resistant”) were grouped together (n=35) and compared to PP/S pts. There was a significant difference in OS (P =.05) but not PFS (P =.12). When pts with 1REL/R were compared to 1REL/S, there was no significant difference in OS (P =.25) or PFS (P =.26).
In this large uniformly treated cohort of HL pts with long-term follow-up, chemoresistance preceding HDT/ASCT was identified as a poor prognostic factor, particularly for PP pts; however, this poor prognostic factor could be partially overcome by HDT/ASCT, resulting in cure in 25–50% of pts across all chemoresistant groups. Importantly, even pts who were double-resistant to both primary and salvage therapy were cured in 27% of cases. HDT/ASCT should therefore be considered in all transplant eligible pts, regardless of responsiveness to salvage chemotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.