Abstract
Abstract 2475
Anti-CD20 antibodies like rituximab are traditionally administered by the intravenous routes although clinical trials by subcutaneous routes have been initiated.
AME-133 is a monoclonal anti-CD20 antibody, has been engineered to be more potent than rituximab. It has 10–20 fold higher binding affinity for the CD20 epitope and approximately 6-fold greater potency in ADCC assays relative to rituximab. The higher potency may provide benefit to patients with low affinity FcgIIIRa and enable subcutaneous administration (patient convenience). In addition, AME-133 has been engineered to be potentially safer than rituximab; AME-133 is humanized to reduce immunogenicity and the CDC activity has been diminished to potentially reduce side effects associated with tumor lysis syndrome. Phase I/II clinical studies of AME-133 in rituximab pretreated/relapsed, patients with low affinity FcγIIIRa, follicular non-Hodgkin's lymphoma (NHL) in the US and in Japan have shown excellent safety profile and response rate (RR) greater than 30%. As a prelude to investigating the clinical efficacy and safety of AME-133 in subcutaneous formulation, a safety/PK study was conducted in cynomolgus monkeys.
The objective of this study was to evaluate the systemic toxicity and pharmacokinetics of AME-133 in cynomolgus monkeys at three different dose levels when administered subcutaneously once each week for 14 consecutive weeks. In addition, the effect of AME-133 on B-cell depletion was evaluated.
A total of 48 naive cynomolgus monkeys (24 male and 24 female) were randomly assigned to four dose groups of AME-133 administered subcutaneously once weekly (0.0 mg/kg (vehicle), 0.6 mg/kg, 1.9 mg/kg and 6.0 mg/kg). Within the dose groups, there were two (6 week and 14 week) dosing schedules. In the 6 week dosing schedule, two-third of animals were sacrificed immediately after 6 weeks, and one-third of animals were sacrificed after 4 to 8 weeks of recovery period. In the 14 week dosing schedule, two-third of animals were sacrificed immediately after 14 weeks, and one-third of animals were sacrificed after 4 to 8 weeks of recovery period.
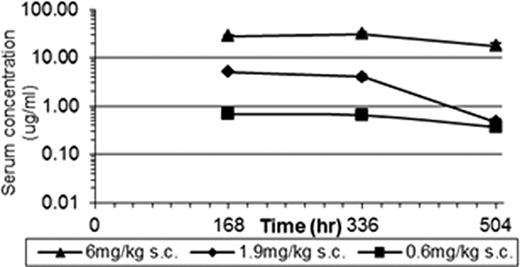
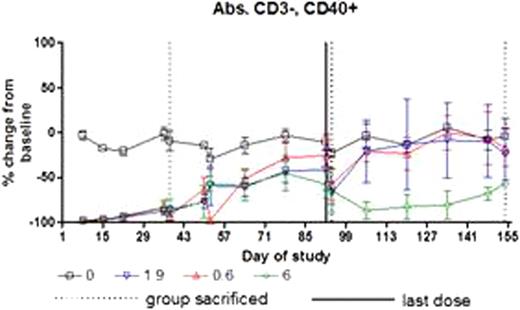
There was no AME-133 related toxicity. All dose levels of AME-133 substantially depleted B-cells in peripheral blood. The extent and duration of B-cell depletion was dose-dependent (Figure 1). B-cell depletion was not affected by gender of the animal.
Serum concentrations of AME-133, 1b. B-cell depletion in peripheral blood.
Serum concentrations of AME-133, 1b. B-cell depletion in peripheral blood.
All dose levels of AME-133 substantially depleted the B-cells in peripheral blood beginning with the first post-dose time point (4 hours after infusion). The mid and high doses resulted in delayed return to normal during recovery in cynomolgus monkeys in 14 week dosing schedule.
This study in cynomolgus monkeys showed AME-133 caused no toxicity, well absorbed and rapidly depleted circulating B-cells when administered subcutaneously. The No Observed Adverse Effect Level (NOAEL) is 6.0 mg/kg, the highest administered dose. These preliminary results justify further investigation of AME-133v administered subcutaneously in follicular lymphoma and other B-cell malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.