Abstract
Abstract 2547
The flow cytometric detection of minimal residual disease (MRD) relies on the differential expression of antigens between normal and leukemic cell populations of similar lineage. In T-cell lymphoblastic leukemia (T-ALL), the principal normal populations from which leukemic blasts must be distinguished are mature T cells and NK cells, as immature T cells are not normally present in peripheral blood or bone marrow. Current methods rely on a relatively small number of antigens, some of which are not stable following therapy. In particular, immature antigens such as TdT and CD99 that distinguish leukemic and mature cells at diagnosis often revert to mature levels after therapy. The subset of T-ALL cases having expression of surface CD3 can be particularly problematic. Consequently, the identification of novel antigens to supplement those currently in use is highly desirable.
We undertook a systematic search for novel antigens capable of distinguishing T-ALL from mature T cell and NK cell populations using a high-throughput flow cytometric screening technique. LyoPlates (Becton-Dickinson) containing antibodies against 242 unique antigenic specificities in a 96 well plate format were used to assay mononuclear cells obtained from 3 normal peripheral blood donors and 9 pediatric patients with T-ALL. The T-ALL cases covered the range of immunophenotypes seen in this disorder. The 9-color assay (1 detection and 8 gating fluorochromes) was performed on an LSRII and was capable of identifying discrete T cell, NK cell and Blast cell subpopulations. Comparison of the median fluorescence intensity of each of the 242 unique antigens identified CD48 as one of the few antigens that showed consistent differential expression between mature T cells and NK cells in comparison to leukemic blasts from T-ALL.
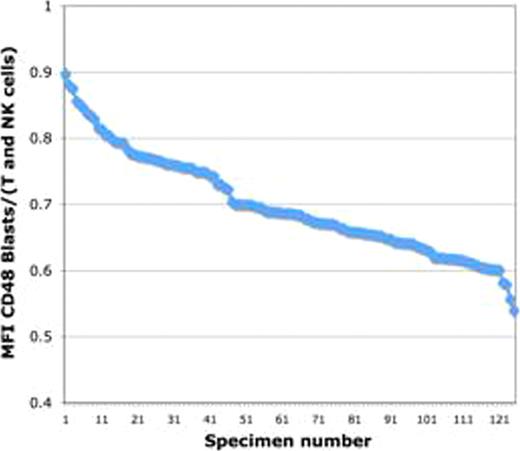
The stability of CD48 expression following therapy was determined by assay of CD48 expression in 126 bone marrow samples obtained at day 29 following induction chemotherapy, representing paired samples from the patients whose pretreatment samples were assayed above. The same 8 color reagent combination was used in conjunction with the standard MRD assayed utilized for the treatment protocol, a two-tube 9 color assay. Of these samples, 50 (39.7%) showed detectable MRD using the standard assay. At end induction, the ratio of CD48 median fluorescence intensity between the leukemic MRD population and normal T and NK cells within the same sample remained abnormal in most cases. It was unchanged in 26% of cases, decreased (i.e. became more aberrant) in 24%, and increased in 50%, but in most of the latter cases remained significantly <1. However, in 5 cases (10%), CD48 increased to the level seen on normal mature T and NK cells. The changes following therapy were due largely to changes in the expression of CD48 on leukemic blasts as the level of expression of normal T and NK cells was more stable. No association between apparent immunophenotypic maturational stage or surface CD3 expression was identified.
We conclude that the expression of CD48 is consistently reduced on leukemic blasts from patients with pediatric T-ALL in comparison to normal mature T and NK cells at the time of diagnosis. Following therapy, the expression of CD48 undergoes modulation, but remains different from mature T and NK cells in 90% of patients. This suggests that CD48 is a useful addition to reagent combinations for the purpose of MRD assessment in pediatric T-ALL.
Wood:BD Biosciences: Research Funding. Borowitz:BD Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.