Abstract
Abstract 2934
Len combined with Dexamethasone (Dex) is becoming a standard treatment in multiple myeloma (MM). As Len is mainly excreted by the kidneys, its dose should be adjusted according to renal function. Current dosing recommendations are based on a study conducted in non-malignant patients (pts) and on modelling/simulations. To assess whether these recommendations are actually valid in MM pts, we conducted a prospective study evaluating pharmacokinetics (PK), safety and efficacy of Len+Dex in pts with various degrees of renal impairment (RI). We also compared the glomerular filtration rate (GFR) estimated either by the Modification of Diet in Renal Disease (MDRD) dosage (GFR/MDRD) or by the Cockroft and Gault (CG) equation (GFR/CG) for determining Len dosage.
37 Caucasian pts (median age 65 yrs) with symptomatic MM who had received ≥ 1 previous line of treatment, were enrolled. All had stable renal function over 4 weeks prior to inclusion. They were divided in 5 groups according to GFR/CG at baseline: group 1, > 80mL/min (N = 10); group 2, ≥ 50 & ≤ 80 mL/min (N = 10); group 3, ≥ 30 & < 50 mL/min (N = 7); group 4, < 30 mL/min (N = 5); group 5, chronic hemodialysis (N = 5). Cast nephropathy and light chain deposition disease were documented by kidney biopsy as the respective cause of RI in 6 and 1 of the 17 pts from groups 3, 4 and 5. Pt characteristics were similar, except for pts in group 4 who were significantly older (p = 0.01). All pts received ≥ 3 cycles of oral Len+Dex (40 mg weekly) regimen from Days 1–21 of each 28-day cycle. Len starting dose was defined according to the current dosing guideline, i.e.: group 1 and 2: 25 mg/d; group 3: 10 mg/d; group 4: 15 mg/qod; group 5: 5 mg/d. Blood samples were collected on a dosing day in the first cycle for PK analyses, as follows: at pre-dose, 0.5, 1, 1.5, 2, 4, 6, 8, 10, 24 h (and 36 and 48 h in groups 3–5) after the Len dose on day 5, and at predose and 2 h (near tmax) after the Len dose on days 9 and 15.
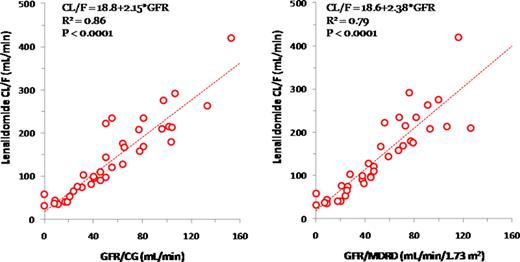
Len clearance was highly correlated to GFR/CG (R2 = 0.86, p < 0.001). Mean Len clearance declined from 239 mL/min in group 1, to 160, 93, 54, and 41 mL/min, and mean terminal half life was prolonged from 3 h to 5, 7, 10, and 24h in groups 2, 3, 4 and 5, respectively. These findings were consistent with those reported for pts with RI due to non-malignant conditions. The average daily AUC values for groups 2–5 were 103–149% of that for group 1 (1794 h*ng/mL). No difference was found in mean plasma Len concentrations at 2 h post-dose between day 9 (Len alone) and day 15 (Len+Dex) across groups. GFR/MDRD and GFR/CG were highly correlated (R2 = 0.85, slope = 0.89) and similarly predicted Len clearance (slope = 2.38 and 2.15, respectively). When the same cutoff values were used for GFR/MDRD and GFR/CG, the % reduction in Len clearance in groups 3–5 compared with the combined groups 1 and 2 was very similar for GFR/MDRD- and GFR/CG-based renal function classifications. However, a dosage discordance between GFR/MDRD and GFR/CG would occur in 5/32 non-dialysis pts. All the 5 pts would be assigned to a lower starting dose according to GFR/MDRD. The estimated resulting AUC levels would have been reduced to 68–94% of the mean AUC in group 1 from the observed 91–238%. These estimated AUC levels would be considered to be in the therapeutic range.
After 3 cycles of Len+Dex, hematological response rate (≥ PR) was 73% (VGPR 27%). Response rates were 70% in pts with < 50ml/min. Renal function remained stable in all pts. A total of 22 serious (≥ grade 3) adverse events (SAEs), including 16 hematological SAEs, occurred in 12 pts, leading to dose reduction in 7 cases. Of these, 2 pts would have been assigned to a lower starting dose according to GFR/MDRD. The frequency of SAEs was not significantly increased in group 5 compared to the other groups (60% vs 45%, p = 0.3). After a median follow-up of 10 months, pts had received a mean number of 7.6 ± 4.3 cycles, and 5 had died, because of MM progression in 4 cases.
The study demonstrated that the effect of RI on the Len PK in MM patients receiving concomitant Dex was similar to that in non-malignant pts receiving Len alone. The recommended dose adjustments achieved the appropriate plasma exposure with similar efficacy and safety across different renal function groups in MM pts. GFR/MDRD and GFR/CG may be interchangeable for determining the Len dosage.
Relationship between Renal Function and Len Clearance
:Comparison of the Predicted and Observed Len AUC at the Recommended Doses
:Comparison of the Predicted and Observed Len AUC at the Recommended Doses
Chen:Celgene Corporation: Employment. Alakl:Celgene Corporation: Employment. Neel:Celgene Corporation: Employment. Jaccard:Celgene Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.