Abstract
Abstract 2936
Almost all patients (pts) with multiple myeloma eventually relapse and remission duration decreases with each regimen. The median Progression Free Survival (PFS) and Overall Survival (OS) in pts with relapsed myeloma refractory to lenalidomide (len) and bortezomib (btz) is poor at 5 and 9 months respectively. A phase 1 study of len plus btz in pts with relapsed or relapsed, refractory MM (RRMM) demonstrated favorable toxicity and promising response and survival further confirmed in a phase 2 study with len, btz and dexamethasone (dex) [RVD]. In this retrospective study, we assessed the efficacy and toxicity profile of RVD therapy for pts with advanced RRMM.
We retrospectively reviewed the records of all pts with RRMM treated with RVD at Princess Margaret Hospital between 03/09 and 05/11. Relapse was defined according to the Uniform International Criteria. Pts were given RVD therapy as previous described by Anderson et al and must have completed at least one cycle of RVD therapy. Primary endpoints were response rate (RR), PFS, OS, and toxicity. Pts discontinued therapy if they experienced PD, no additional benefit or unacceptable toxicity. Definitions of response and progression were used according to the EBMT modified criteria with a category of very good partial response (VGPR). To examine variables independently prognostic for PFS and OS, multivariate Cox analysis was performed. Differences in continuous variables between groups were compared using Mann-Whitney or Kruskal-Wallis tests. Survival curves were constructed according to the Kaplan-Meier method and compared using the log rank test.
Clinical characteristics of patients with RRMM treated with RVD
| Clinical characteristic N=30 . | Median . | Range . | % . |
|---|---|---|---|
| Age | 57 | 37-76 | |
| Male | 46.7% | ||
| Female | 53.3% | ||
| Hemoglobin (g/L) | 105 | 71-155 | |
| Creatinine (mmol/L) | 99.9 | 36-383 | |
| Beta-2 microglobulin (mmol/L) | 280 | 119-1440 | |
| Lactate dehydrogenase (U/L) | 181 | 89-255 | |
| IgG | 56.6% (17) | ||
| IgA | 23.3% (7) | ||
| IgM | 3.3% (1) | ||
| Light Chain | 16.6% (5) | ||
| Kappa (mg/L) | 400 | 5.3-3460 | 63.3% (19) |
| Lambda (mg/L) | 514 | 5.1-5300 | 36.7% (11) |
| Kappa | |||
| Lambda | |||
| *BMPC | 57% | 6-95% | |
| M-spike serum (g/L) | 30 | 0-77 | |
| M-spike urine (g/d) | 0.89 | 0-7.9 | |
| Prior therapies | 3 | 1-6 | |
| ASCT | 83.3% (25) | ||
| Thal | 60% (18) | ||
| Len | 73.3% (22) | ||
| Btz | 80% (24) |
| Clinical characteristic N=30 . | Median . | Range . | % . |
|---|---|---|---|
| Age | 57 | 37-76 | |
| Male | 46.7% | ||
| Female | 53.3% | ||
| Hemoglobin (g/L) | 105 | 71-155 | |
| Creatinine (mmol/L) | 99.9 | 36-383 | |
| Beta-2 microglobulin (mmol/L) | 280 | 119-1440 | |
| Lactate dehydrogenase (U/L) | 181 | 89-255 | |
| IgG | 56.6% (17) | ||
| IgA | 23.3% (7) | ||
| IgM | 3.3% (1) | ||
| Light Chain | 16.6% (5) | ||
| Kappa (mg/L) | 400 | 5.3-3460 | 63.3% (19) |
| Lambda (mg/L) | 514 | 5.1-5300 | 36.7% (11) |
| Kappa | |||
| Lambda | |||
| *BMPC | 57% | 6-95% | |
| M-spike serum (g/L) | 30 | 0-77 | |
| M-spike urine (g/d) | 0.89 | 0-7.9 | |
| Prior therapies | 3 | 1-6 | |
| ASCT | 83.3% (25) | ||
| Thal | 60% (18) | ||
| Len | 73.3% (22) | ||
| Btz | 80% (24) |
BMPC, Bone marrow plasma cells
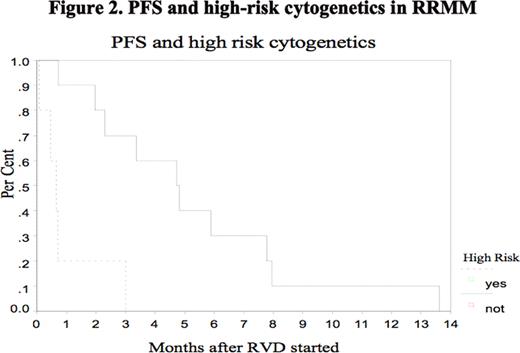
In conclusion, RVD is active and well tolerated in pts with RRMM, including pts who have received prior len, btz, thal and ASCT but PFS is short at 3.9 months in this highly advanced disease group of patients. We question whether response is dependent on recognized risk factors such as adverse cytogenetics.
Jimenez-Zepeda:J & J: Honoraria. Reece:Bristol, Meyers, Squibb: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Johnson&Johnson: Research Funding; Merck: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Millennium: Research Funding; Amgen: Honoraria. Kukreti:Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.