Abstract
Abstract 2951
Everolimus (RAD001) is an inhibitor of MTORC1, a component of the Akt-MTOR pathway which regulates growth and survival of lymphoplasmacytic cells in Waldenstrom's Macroglobulinemia (WM). Everolimus also exhibits activity in WM patients with relapsed/refractory disease (Ghobrial et al, JCO 2010; 28 :1408–14). We therefore initiated this multicenter, prospective study to delineate the efficacy and tolerability of Everolimus as primary therapy in WM.
WM patients with symptomatic disease, adequate organ function, who were not previously treated, and who did not have symptomatic hyperviscosity were eligible for this study. Intended therapy consisted of 10 mg of oral Everolimus administered daily, with sequential dose de-escalation to 7.5 mg daily, 5 mg daily, and 5 mg every other day permitted for toxicity. Patients were treated until progression or unacceptable toxicity. Patients were encouraged to use 5 mL of an oral dexamethasone solution (0.5 mg/5mL) to swish and spit up to 4 times daily for prevention of oral ulcerations associated with Everolimus. Study participants were assessed monthly for the first 3 months, and thereafter every 3 months which included a physical examination, complete blood counts, chemistries, and serum IgM monitoring. Bone marrow biopsies and aspirations were performed at baseline, at months 6 and 12, and as required for response assessment.
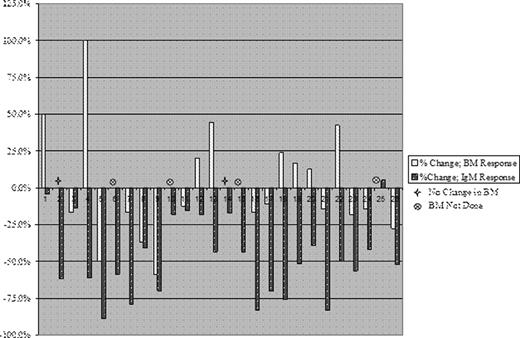
Thirty-three patients were enrolled on this prospective, multicenter study and are evaluable for response. Median baseline characteristics for all patients are as follows: Age 62 (range 41–80 years); Hematocrit 31.3% (range 24.5–45.7%); Hemoglobin 10.8 (range 7.8–15.7 g/dL); serum IgM 4, 440 (range 959–10, 256 mg/dL), with 23 (69.7%) patients demonstrating an IgM level ≥3, 000 mg/dL; serum M-protein 2.60 g/dL (range 0.31–5.31 g/dL), B2M 3.0 mg/L (1.6–6.7 mg/L). The median baseline bone marrow disease burden was 70% (range 7.5–95%), and 21 patients (63.6%) demonstrated adenopathy or splenomegaly by CT scans at baseline. At best response, serum IgM levels declined from 4, 440 to 1, 925 (p<0.0001), and serum M-protein decreased from 2.60 to 1.50 g/dL (p<0.0001). The median time to best serum IgM response was 3 months (range 0.6–15 months). Median hematocrit and hemoglobin levels declined modestly from 31.3% to 30.6% (p=0.057) and 10.8 to 10.4 g/dL (p= 0.1059), respectively. Twenty-two patients are evaluable for response by both bone marrow biopsy and IgM level at 6 months, at which time bone marrow disease burden remained unchanged with a median of 65% involvement (range 10–95%; p=0.3595). The best overall response rate utilizing consensus criteria was 66.7% (14 Partial Responses, 8 Minor Responses, and 11 Stable Disease), for a major response rate of 42.4%. However, discordance between serum IgM levels upon which consensus criteria for response are based, and bone marrow disease response were common and complicated response assessment. At 6 month assessments, 10 of 22 (45.5%) patients for whom both serum IgM and bone marrow assessments were performed, discordance between serum IgM levels and bone marrow disease involvement were observed. Among these patients, 2 had no change, and 8 had increased bone marrow disease involvement despite decreases in serum IgM levels. Grade ≥2 hematologic and non-hematologic toxicities possibly, probably or definitively associated with Everolimus included anemia (n=8, 24%), thrombocytopenia (n=5, 15%), neutropenia (n=5, 15%), hyperglycemia (n=2, 6%), oral ulcerations (n=7, 21%), pneumonitis (n=5, 15%), fatigue (n=4, 12%), rash (n=2, 6%), and cellulitis (n=2, 6%). With a median follow-up of 9 months (range 0–18 months), 15 patients remain on study. Reasons for study discontinuation included non-response or disease progression (n=11), unacceptable toxicity (n=6, including 5 for pneumonitis and 1 for neutropenia), and loss of follow-up (n=1).
Everolimus is active in the primary therapy of WM, with rapid reductions observed in serum IgM levels in most patients. Serum IgM discordance to underlying bone marrow disease burden is common, and serial bone marrow assessments are important for response monitoring in WM patients receiving Everolimus.
Changes in bone marrow disease burden versus serum IgM levels at 6 months following initiation of Everolimus in previously untreated WM patients.
Changes in bone marrow disease burden versus serum IgM levels at 6 months following initiation of Everolimus in previously untreated WM patients.
Treon:Millennium: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Genentech: Honoraria. Eradat:Millennium: Speakers Bureau; Genentech, A Roche Company: Speakers Bureau. Matous:Seattle Genetics, Inc.: Research Funding; Celgene: Speakers Bureau; Cephalon: Speakers Bureau; Millennium: Speakers Bureau. Anderson:Millennium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Acetylon: Equity Ownership. Ghobrial:Noxxon: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.