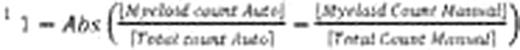Abstract
Abstract 3001
The hematopoietic colony-forming-cell (CFC) assay is a valuable tool to assess the potency of cell products (umbilical cord blood, apheresis products, bone marrow) for hematopoietic stem cell transplantation and for toxicity screening in therapeutic drug development. However, the manual colony enumeration that has been required is subjective and time consuming even for experienced users. This subjectivity limits the accuracy of the assay and contributes to a high degree of inter-laboratory variability. To reduce this variability, STEMCELL Technologies has developed an imaging and analysis system (STEMvision™) for automation of CFC assay colony enumeration. We have previously shown results validating the instrument for use in cord blood (CB) cell assays (Wognum et al. 37th Annual Meeting of the European Group for Blood and Marrow Transplantation, Hamburg, Germany 2011). Here we additionally present recent results comparing automated and manual colony counting for human mobilized peripheral blood (mPB), and bone marrow (BM).
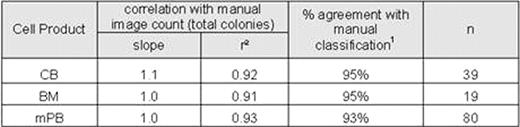
Samples of CB, mPB, and BM mononuclear cells were inoculated into semi-solid culture media (Methocult™ H4034, H4434, and H4435) and plated into special meniscus-free culture plates (SmartDish™). After 14 days in culture, automated colony counts were obtained from each sample using algorithms specifically optimized for each type of product (CB, mPB, and BM). The same cultures were then manually enumerated by 2–5 operators using the standard microscope method (microscope counts) and by enumerating colonies on the STEMvision™ images (image counts).
Variability of colony counting was also significantly reduced with the STEMvision™ instrument. We found that for multiple independent measurements of a given sample, the coefficient of variance (CV) of the normalized counts was 11% for the microscope counts (2–5 different operators), 7.9% for the image counts (2–5 different operators) and 4.4% for the automated counts (2–5 different instruments). The low CV for the automated counts was not operator dependent: for 6 samples analyzed by 6 operators using a single instrument, the CV for normalized total colony counts was 4.3%.
Automated colony counting with the STEMvision™ instrument has thus been shown to be highly correlated to manual scoring methods for the most common hematopoietic stem cell transplantation products(CB, mPB, and BM). In addition, automation of the assay analysis significantly reduced the variability across multiple operators relative to manual counting methods. As a result, STEMvision™ has the ability to improve standardization of the CFC assay analysis through reduced intra- and inter-lab variability. We have reported previously on the internal and independent multi-center validation of this automated system for CB products. Rigorous validation of for BM and mPB cell products is currently in progress.
In addition to providing standardization, this instrument reduces the time required for the assay readout and provides a means of permanent archiving of colony assay images. In the future, the image analysis output will provide quantitative information related to colony morphology that is not easily obtained by manual analysis (eg. colony size, density, symmetry). Such information would enable automated assessment of hematopoietic toxicity.
Egeler:Stemcell Technologies: Employment. Wognum:Stemcell Technologies: Employment. Grande:Stemcell Technologies: Employment. Yuan:Stemcell Technologies: Employment. Woodside:Stemcell Technologies: Employment. Thomas:Stemcell Technologies: Employment, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.