Abstract
Abstract 3078
Adding rituximab to induction therapy has improved overall survival in patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) after high dose chemotherapy followed by autologous stem cell transplantation (AuSCT) to 50–60%. However, there is still room for improvement. Addition of 90Yttrium ibritumomab tiuxetan prior to the BEAM conditioning regimen seems feasible and results in promising data with respect to disease free and overall survival in high risk DLBCL patients even when treated with rituximab containing induction therapy. At the VU University Medical center, rituximab was added to (re-)induction therapy starting July 2001. From 2006 we started to add 90Yttrium ibritumomab tiuxetan (Zevalin®) to BEAM (Z-BEAM) in high risk DLBCL patients. In this retrospective analysis we compare outcome of Z-BEAM versus BEAM, both followed by AuSCT.
All high risk DLBCL patients consolidated with high dose (radio-immuno) chemotherapy and AuSCT in CR or PR after rituximab-containing induction therapy were included. High-risk DLBCL was defined as either relapsed or refractory DLBCL or as histological transformation of indolent NHL. AuSCT was preceded by BEAM conditioning and 90Yttrium ibritumomab tiuxetan (0, 4 mCi/kg, max 32 mCi, starting 2006: Z-BEAM group) or by BEAM only (BEAM group). EFS and OS were estimated using the Kaplan-Meier method and compared using the log rank test.
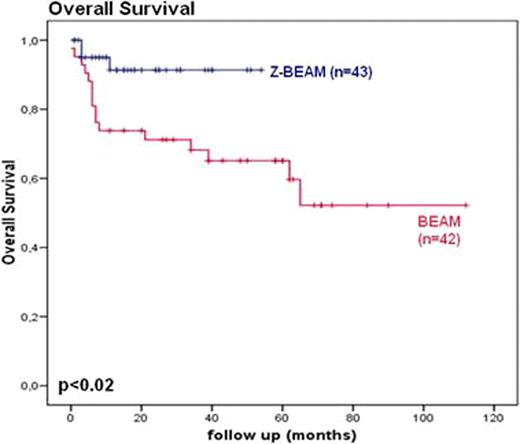
43 patients received Z-BEAM and 42 patients received BEAM conditioning. Median age was 56 and 52 years respectively. No significant differences in disease characteristics were seen. Median follow up (range) was 15 months (6–54) and 39 months (0–112) respectively. Overall survival was significantly better in the Z-BEAM group compared with the BEAM group (p=0.02) with an estimated 2 year overall survival of 90% vs. 65%. (fig 1.) In the first 2 years of follow up 7 patients in the Z-BEAM group relapsed compared to 11 in the BEAM group, this did not reach significance (p=0.09). Median time to recovery of neutrophils and thrombocytes was not significantly different. Moreover, there was no significant difference in TRM (no TRM in the Z-BEAM group versus 2 patients in the BEAM group). Patients who relapsed, in both groups, were able to receive re-induction chemotherapy and, if indicated, allogeneic SCT without being compromised by decreased bone marrow reserve or non haematological toxicities.
Adding 90Yttrium ibritumomab tiuxetan to the BEAM conditioning regimen preceding AuSCT leads to a significant improvement in overall survival in high risk DLBCL patients, even if they have received rituximab during (re)induction.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.