Abstract
Abstract 3314
For congenital hemophilia patients who develop inhibitors to factors VIII or IX, the management of bleeding episodes can be complex, and often requires use of bypassing agents (BPA) such as rFVIIa (NovoSeven® RT). While some have reported on the variability in responsiveness to BPAs between patients, with the implication that some patients are more or less responsive to particular agents, few trial or registry data sets have sufficient long-term data to capture the intra-patient variability in dosing for specific bleed types or locations to identify if treatment is individualized on a patient basis or instead on a bleed-by-bleed basis by the hematologist and patient/caregiver. The HTRS Registry is a multi-center, longitudinal database of bleeding episodes and treatment patterns for bleeding disorder patients, with a particular focus on the treatment of acute bleeding episodes in inhibitor patients.
The HTRS registry recorded data between January 2004-March 2011 for 2,053 bleeding episodes in 134 inhibitor patients. From these data, we identified US patients with 75 or more rFVIIa-treated bleeding episodes and analyzed for intra-patient variability using descriptive statistics and graphical representations.
5 patients (3 hemophilia A from 2 centers, 2 hemophilia B from 1 center) were identified with 568 rFVIIa-treated bleeds (28% of total recorded). Patients were White and Non-Hispanic, and ranged in age from 2.2 to 13.7 years old at the time of first reported bleed (2000–2005) and from 6.2 to 17.6 years old at the time of last reported bleed (2004–2008). Patients had 95–162 documented bleeds (mean 113.6) over 38–98 months (mean 62.5), with between 1 and 2.7 bleeds per month (mean 2.0). Median total dose per bleeding episode and interquartile ranges (IQR, 25%-75%) are shown below.
| Patient number . | rFVIIa Trx Bleeds . | Statistic . | Total rFVIIa Dose Per Episode (mcg/kg) . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | All Bleeds . | Traumatic . | Spontaneous . | Joint . | Muscle . |
| A | 162 | Median | 663.5 | 816 | 640 | 640 | 692 |
| IQR | 480–940 | 524–1000 | 469–906 | 480–940 | 465–927 | ||
| B | 106 | Median | 640 | 595 | 640 | 600 | 745 |
| IQR | 470–1000 | 427.5–867 | 480–1000 | 414–820 | 540–1140 | ||
| C | 103 | Median | 630 | 855 | 630 | 675 | 790 |
| IQR | 370–1109 | 483–1363 | 400–900 | 450–1131 | 485–1095 | ||
| D | 102 | Median | 570 | 600 | 600 | 600 | 540 |
| IQR | 360–960 | 360–990 | 424 –960 | 480–960 | 360–1485 | ||
| E | 95 | Median | 711 | 474 | 724.5 | 774 | 430 |
| IQR | 273–840 | 354–592.5 | 297–840 | 492–840 | 258–765 | ||
| Patient number . | rFVIIa Trx Bleeds . | Statistic . | Total rFVIIa Dose Per Episode (mcg/kg) . | ||||
|---|---|---|---|---|---|---|---|
| . | . | . | All Bleeds . | Traumatic . | Spontaneous . | Joint . | Muscle . |
| A | 162 | Median | 663.5 | 816 | 640 | 640 | 692 |
| IQR | 480–940 | 524–1000 | 469–906 | 480–940 | 465–927 | ||
| B | 106 | Median | 640 | 595 | 640 | 600 | 745 |
| IQR | 470–1000 | 427.5–867 | 480–1000 | 414–820 | 540–1140 | ||
| C | 103 | Median | 630 | 855 | 630 | 675 | 790 |
| IQR | 370–1109 | 483–1363 | 400–900 | 450–1131 | 485–1095 | ||
| D | 102 | Median | 570 | 600 | 600 | 600 | 540 |
| IQR | 360–960 | 360–990 | 424 –960 | 480–960 | 360–1485 | ||
| E | 95 | Median | 711 | 474 | 724.5 | 774 | 430 |
| IQR | 273–840 | 354–592.5 | 297–840 | 492–840 | 258–765 | ||
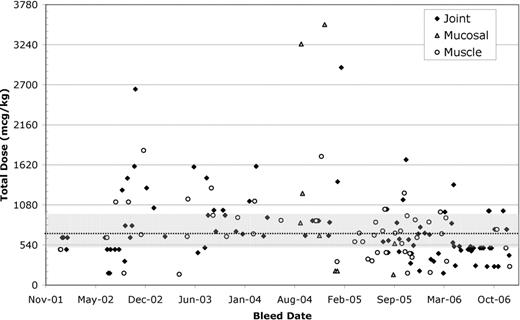
Median total dose per episode for all bleeds varied from 570 to 711 mcg/kg, with the minimum reported dose of 50 mcg/kg and maximum of 43,200 mcg/kg. There was some variability but no consistent pattern across patients when comparing median doses for traumatic, spontaneous, joint, muscle and mucosal bleeds. The ratios of IQR/median and range/median total dose were also variable across patients and often across types within patients.
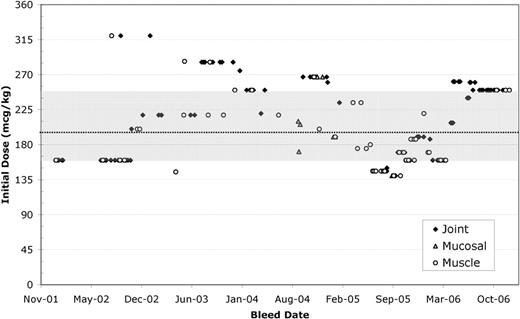
The variability in total dose across bleed types/locations can also be demonstrated graphically as in the figures below (patient A, IQR - shaded, median – dotted line). Furthermore, initial doses were consistently above US package insert recommendations of 90 mcg/kg and high initial doses (>250 mcg/kg) were seen throughout 2002–2006.
While registry data are subject to a variety of biases, they are able to capture real world data across patient populations that allows for assessment of variability between patients. In the case of the longstanding HTRS registry, we have the ability to also examine in a limited way differences in dosing within 5 individual patients across a 3–8 year period of childhood and adolescence. Surprisingly, or perhaps not so surprisingly, these exploratory analyses seem to indicate that treatment may be individualized by the hematologist (and hemophilia treatment center) and patient/caregiver for every bleed, and that even in individuals with many spontaneous, traumatic, or joint bleeds, treatment varies between mild bleeds treated with as little as one dose and severe bleeds or surgeries requiring prolonged treatment. Given the challenges of documentation of home treatment within medical records and consequently limited information available for assessment in registries, further analysis of co-variates that can account for these intra-patient variations (e.g. increasing frequency of target joint bleeds, extent of trauma, concurrent activities/sports/camp) will require prospective observational diary or registry studies that can capture additional information.
Neufeld:Novo Nordisk Inc.: Consultancy, Research Funding. Off Label Use: rFVIIa (NovoSeven) is approved for the treatment of bleeding episodes in congenital hemophilia with inhibitors; doses shown in this abstract might differ from the US prescribing information. Cooper:Novo Nordisk Inc.: Employment.