Abstract
Abstract 3815
Azacitidine (AZA) is approved for the treatment of patients with higher-risk myelodysplastic syndromes (MDS) and increases median overall survival (OS) by 9.5 months compared with conventional care regimens (CCR). However, the median OS for AZA-treated patients in this population is only two years (Fenaux, 2009, Lancet Oncol). Biomarkers predictive of maximal clinical benefit with AZA would allow identification of those patients ideally suited for AZA therapy. One of the molecular mechanisms of AZA activity is hypomethylation of DNA. Previous evaluation of DNA methylation of 5 genes (CDKN2B [p15], SOCS1, CDH1 [E-cadherin], TP73, and CTNNA1 [α-catenin]) in baseline bone marrow (BM) samples of MDS patients in the Phase 3 AZA-001 trial demonstrated that increased DNA methylation in pre-treatment baseline BM was associated with worse OS, and patients with lower levels of methylation derived the best OS benefit from AZA therapy (Herman, 2009, AACR).
In the present study, we expanded our evaluation of DNA methylation patterns in the baseline BM of MDS patients from the AZA-001 trial to identify a DNA methylation-based predictive signature of OS in AZA-treated patients. DNA methylation levels of 27,578 genomic loci were determined in pre-treatment unpurified BM aspirates of 129 patients (AZA [n=59] and CCR [n=70]) using Illumina Infinium Methylation27 Beadarray. The data were randomly divided into a training data set (n=95: AZA [n=38] and CCR [n=57]) and a validation data set (n=34: AZA [n=21] and CCR [n=13]). A DNA methylation signature predictive of OS in AZA-treated patients was identified using uniCox algorithm (Tibshirani, 2009, Stat Appl Genet Mol Biol) first in the training data and then re-evaluated in the validation data. The influence of the signature on OS was assessed using Cox proportional hazards models. All statistical analyses were carried out in R (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
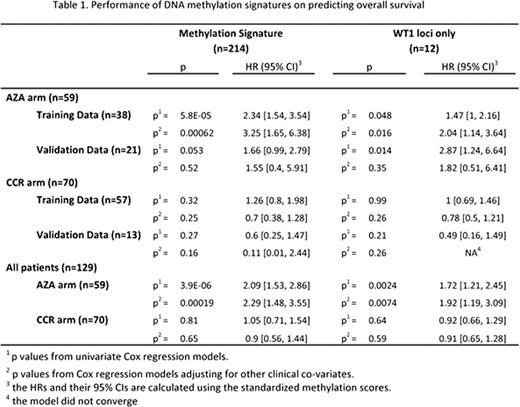
A baseline DNA methylation signature predictive of OS was identified based on the training set of 38 AZA-treated patients and re-evaluated in the validation set of 21 AZA-treated patients. The signature contains 214 genomic loci representing 187 genes, including WT1, CDH1, and CDKN2B. A DNA methylation score was calculated for each patient as the weighted average of the DNA methylation levels of these 214 genomic loci, with weights optimized in the training data set. As expected, in the training data set, the DNA methylation score was significantly (hazard ratio [HR]=2.34 [95% confidence interval: 1.54, 3.54]; p=5.8E-5) associated with OS and remained significant (HR=3.25 [1.65, 6.38]; p=0.00062) in a multivariate analysis, adjusting for the clinical co-variates: sex, cytogenetic score, ECOG grade, and baseline RBC transfusion status. The DNA methylation score was not predictive of OS in patients treated with conventional care (p=0.81), demonstrating the specificity of this marker to AZA therapy. When applied to the validation data set, the association of the DNA methylation score with OS remained marginally significant (HR=1.66 [0.99, 2.79]; p=0.053) in a univariate analysis, but became non-significant (p=0.52) in a multivariate analysis. For the majority of the loci in the signature, lower DNA methylation correlated with better OS. Interestingly, DNA methylation of WT1 loci alone was predictive of OS in both univariate (HR=1.47 [1.00, 2.16]; p=0.048) and multivariate (HR=2.04 [1.14, 3.64]; p=0.016) analyses of AZA-treated patients in the training data set. DNA methylation of WT1 loci remained a significant (HR=2.87 [1.24, 6.64]; p=0.014) predictor of OS in the validation data set in a univariate analysis, but became non-significant (p=0.35) in a multivariate analysis. As with the signature containing 214 loci, DNA methylation of WT1 loci was not predictive of OS in patients treated with conventional care (p=0.64). All performance results are summarized in Table 1.
We have identified a DNA methylation signature that correlates with OS in the unpurified pre-treatment BM aspirate samples of AZA-treated higher-risk MDS patients. We have also demonstrated that DNA methylation of WT1 loci alone was predictive of OS. As these markers could be used to make therapeutic choices for patients with MDS, their predictive values warrant further validation in a larger independent data set.
Shi:Celgene Corporation: Employment, Equity Ownership. Luo:Celgene Corporation: Employment, Equity Ownership. Lucy:Celgene Corporation: Employment, Equity Ownership. Beach:Celgene Corporation: Employment, Equity Ownership. MacBeth:Celgene Corporation: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.