Abstract
Chemoimmunotherapy, namely the combination of fludarabine, cyclophosphamide and rituximab (FCR), results in a higher response rate and a longer progression-free survival in patients with chronic lymphocytic leukemia (CLL). There is also evidence coming from several historical comparisons, observational, and epidemiological studies and, above all, a large randomized clinical trial (German CLL Study Group – Hallek M et al, Lancet 2010) that FCR prolongs survival. Since additional randomized studies to validate the superiority of FCR over older therapies in CLL are difficult to envisage, other approaches to confirm this observation are worth to be considered.
The aim of this study was to ascertain whether chemoimmunotherapy given at any time over the course of the disease, independently of the treatment phase, prolonged survival in a group of unselected patients with CLL from the Hospital Clínic of Barcelona.
Out of 1042 consecutive patients diagnosed and followed-up from 1980 to December 2010, we selected 484 patients who received at some point of their disease evolution: (1) alkylating agents and no purine analogs (PA) nor rituximab (R) (no PA no R) (n=211; of which 67 < 65 years). (2) purine analogs but no R (PA) (n=159). (3) PA plus rituximab (PA+R) (n=114). Clinical information (age, sex, Binet stage) and laboratory characteristics (β2-microglobulin, CD38, ZAP-70, genomic alterations, IGHV mutational status) at disease presentation, treatment, and follow-up was obtained from a database prospectively managed at our institution from the 1970s onwards. All treated patients were included in the analysis regardless of the number of cycles of therapy given and independently of whether they had participated in clinical trials or not.
The median age (range) of the whole series (n=340, 222M/118F) was 56 (24–84) years. The three groups were well balanced for key clinical characteristics such as age, sex, and Binet stage at treatment. It should be noted that patients older than 65 years were initially excluded from the analysis to avoid a bias in the results due to the general worse prognosis of older patients. On the other hand, patients from the PA and PA+R groups presented poorer risk prognosis factors such as a higher expression of CD38 (p=0.006), ZAP-70 (p=0.052), and adverse cytogenetic abnormalities (p=0.026) or unmutated IGHV genes (p=0.005) than those in the no PA no R group.
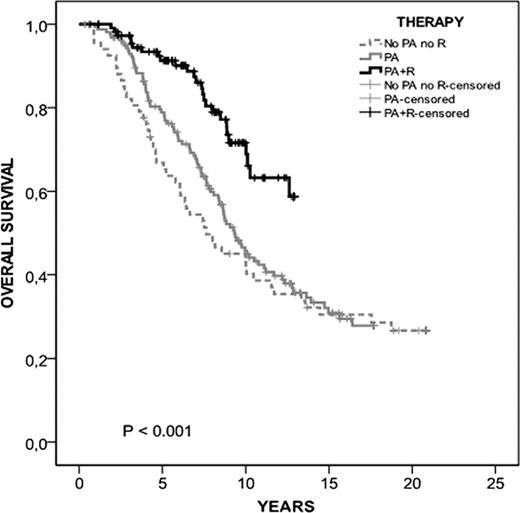
After a median follow up of 9.4 years (range, 0.3–21), 148 (44%) patients remain alive. At 10 years, the overall survival of the PA+R group was 65% (95% CI, 53–77%) compared with 43% (35–51%) and 43% (31–54%) for the no PA no R and PA groups, respectively (p<0.001) (Figure 1).
When all patients who did not receive PA or R were included in the study (n=211), the statistically significant difference was, not surprisingly, maintained (data not shown). In addition, patients from both the PA group and the PA+R group had poorer prognostic features. These data are in keeping with a conservative therapeutic approach in patients with low-risk disease and a poorer risk of patients treated with PA with or without R. This is also an additional argument in favor of the effectiveness of chemoimmunotherapy in high-risk patients.
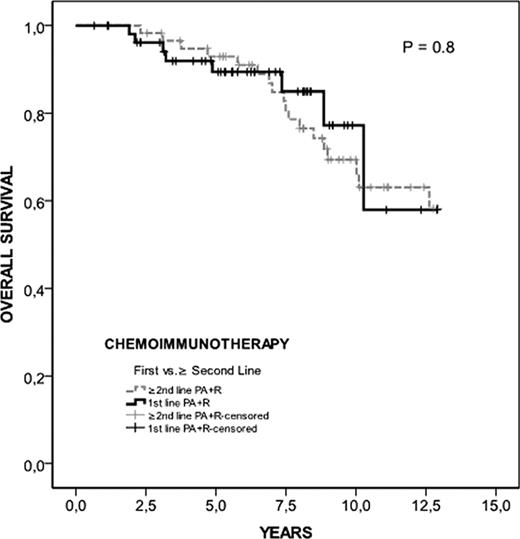
Interestingly, when survival of patients treated with PA+R was analyzed according to the time at which treatment was administered (first line, n=55 vs. ≥ second line, n=59), no differences were observed (p= 0.8) (Figure 2)
Chemoimmunotherapy prolongs survival of patients with CLL. Moreover, this effect could be independent of the phase of the disease at which chemoimmunotherapy is given.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.