Abstract
Abstract 4014
In hematopoietic stem cell transplantation, most immunosuppressive strategies (e.g., using calcineurin inhibitors or corticosteroids) decrease graft-versus-host disease (GVHD) rates, but also impair pathogen- and cancer-specific T cells, increasing treatment-related mortality. Consequently, a key goal of transplant immunology is the identification of more selective inhibitors of alloreactivity. Recent murine studies have shown that alloreactive T cells mediating GVHD reside primarily in naïve and early memory T cell compartments (Anderson BE et al. J Clin Invest 2003;112:101–108, Chen BJ et al. Blood 2004;103:1534–1541, Zheng H et al. J Immunol 2009;182:5938–5948). In contrast, human virus-specific T cells are more differentiated (Gamadia LE et al. Blood 2001;98:754–761). We recently demonstrated that the RAS/MEK/ERK pathway is preferentially activated in naïve and early memory T cells, relative to late memory T cells (Kim TK, submitted). This pathway is activated in a murine experimental GVHD (Lu SX et al. Blood 2008; 112: 5254–5258). Therefore, we hypothesized that MEK inhibition would prove to be a more selective immunosuppressive strategy, suppressing alloreactive but not virus-specific T cells.
Using PBMC from healthy donors, we assessed the effects of several MEK inhibitors or the calcineurin inhibitor (CNI) tacrolimus on functional T cell responses using flow cytometry and/or western blotting. Using single-cell cytometric methods, we examined intracellular phosphorylation of ERK1/2 in response to PMA and Ionomycin. Alloreactivity induced by allogeneic monocyte-derived dendritic cell (DC) stimulation was also investigated. CMV-specific functional T cell responses following stimulation using pp65 pentadecapeptide pools were assessed using cytokine flow cytometry. To assess selectivity of immunosuppressive agents, we examined the effects of CNI and MEK inhibitors, independently and in combination, on alloreactivity and CMV-specific T cell responses.
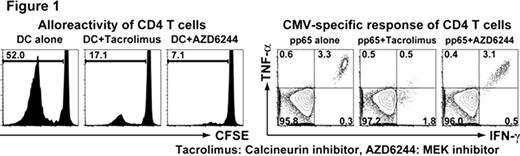
We first confirmed that multiple RAS/MEK/ERK family proteins were differentially expressed with lymphocyte maturation, suggesting that MEK inhibitors would preferentially suppress naïve and early memory T cells. Consistent with this prediction, ERK1/2 was preferentially phosphorylated in naïve and early memory T cells stimulated with PMA/Ionomycin, relative to intermediate and late memory T cells. When we examined the effects of targeted T cell inhibition on alloreactive and virus-specific T cell responses, we confirmed that: 1) MEK inhibitors blocked phosphorylation of ERK1/2 in CD4 and CD8 T cells stimulated with PMA/Ionomycin at 100nM to 10μM; 2) Alloreactivity of T cells was suppressed as effectively by MEK inhibitors as by tacrolimus (see Figure 1, left panels). In addition, functional differentiation of naïve T cells was also suppressed. Furthermore, MEK inhibition and calcineurin inhibition worked synergistically and completely shut off alloreactivity. 3) MEK inhibition suppressed cytokine production in naïve and early memory T cells, but not in late memory T cells, while calcineurin inhibition suppressed all differentiation subsets. 4) At doses effectively suppressing DC-stimulated alloreactivity, MEK inhibition spared CMV-specific T cells, in contrast to tacrolimus (p <0.01) (see Figure 1, right panels).
MEK inhibitors suppress T cells in a memory stage-dependent manner, suggesting a novel paradigm to achieve selective immunity in the transplant setting. MEK inhibitors abrogate alloreactivity while sparing the function of CMV-specific CD4 and CD8 T cells. These data suggest that MEK inhibitors may serve as a new class of agents with potential activity in GVHD, facilitating selective inhibition of alloreactivity by targeting T cells in a memory stage-dependent manner.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.