Abstract
High risk acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) may be amenable to immunotherapy with adoptive transfer of chimeric antigen receptor (CAR) T cells. However, persistence and generation of central memory (CM) are essential for ongoing clinical responses (Morgan et al Science 2006). Our prior work showed that the carbohydrate antigen LewisY (LeY) is expressed in 46% of AML patients (n=33) to varying degrees, making it a suitable and novel target for CAR therapy. In our phase I study, we have assessed the trafficking, persistence and functional capacity of CAR-T cells transduced with an anti-LeY chimeric receptor gene in high-risk patients with LeY positive AML or MDS.
The CAR comprised extracellular humanized scFv recognizing the LeY Ag, linked to an extracellular CD8 hinge region, a transmembrane and cytoplasmic CD28 and CD3 zeta signalling domain. The humanized anti- LeY scFv-CD28-ζ receptor vector was produced as described previously (Westwood J et al PNAS 2005). T cells, harvested from the patient, were transduced with LeY scFv-CD28-ζ pSAMEN retroviral vector. Bulk transduced T cells (LeY-T) were re-infused into the patient after lymphodepleting fludarabine chemotherapy. AML immunophenotyping and cytogenetics were used to monitor minimal residual disease (MRD). PB and BM samples were collected prior to and post CAR-T adoptive transfer. An optimized PCR assay (sensitivity 1:1e5) for the presence of the LeY transgene (TG) was performed on genomic DNA extracted from each sample. To measure both CAR-T cell functional polarization and persistence of CAR expression, PB or BM cells were co-cultured with a cell line expressing LeY (OVCAR-3), in the presence or absence of MHC class I or II blocking antibodies. Culture supernatant was collected at 24 hours and assessed by Luminex assay for Th1 (IFN-g, IL-2), Th2 (IL-4, IL-10), pro-inflammatory (IL-6, IL-17 and TNF) cytokines and TGF-b.
Five AML patients have been enrolled to date. Four patients have had sufficient CAR-T cells generated to provide doses of 0.5 × 109 −1.3 × 109T cells (transduced T cells: 8 – 30% of total T-cells]) with a viability of 96.1– 98.5%. 2 patients (01 and 04) had progressive disease 1 and 2 months after infusion. 2 patients (02 and 05) remain in morphologic remission with stable cytogenetic MRD, 3 and 14 months post infusion.
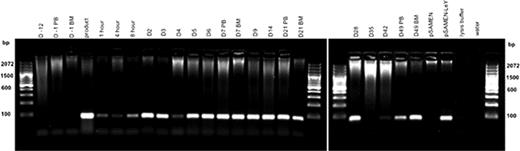
Patients 01, 02 and 04 all had LeY TG in PB and BM samples at all time points measured, up to day 49 in patient 01 (Figure 1) and up to 10 months in patient 02. In patient 04 LeY TG was also detected in a skin biopsy (day +6) of leukemic skin infiltrate.
Patient 01 Genomic DNA was extracted from PB and BM samples and the presence of LeY tg (∼100 bp) assessed by PCR.
Patient 01 Genomic DNA was extracted from PB and BM samples and the presence of LeY tg (∼100 bp) assessed by PCR.
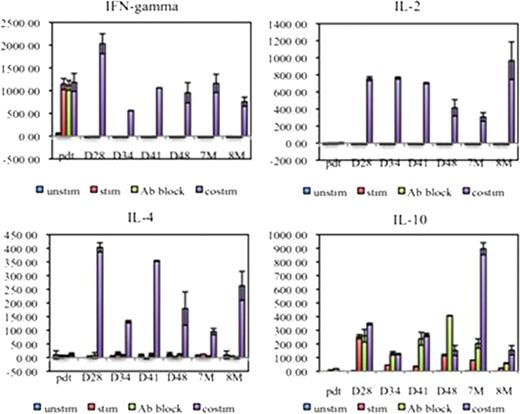
Co-culture supernatant cytokine analysis showed LeY-T cells from patient 01, 02 and 04 secreted high levels of IFN-g (805.5 ± 198.9 pg/ml) and low levels of IL-2 (3.14 ± 0.65 pg/ml) prior to adoptive transfer. In contrast, post adoptive transfer, co-culture with OVCAR-3 cells, and MHC class I and II blocking antibody, induced these PB LeY-T cells to secrete IL-4 (24.13 ± 12.47pg/ml) and IL-10 (9.35 ± 0.44 pg/ml); no IFN-g or IL-2 was detected. This Th2 polarization of CAR-T cells was shown in PB samples taken at days 5 to 28 (patient 01), day 28 to 8 months (patient 02) and day 1 to day 28 (patient 04) post-adoptive transfer (Figure 2). In addition, when Th1/Th2 cytokines were measured in patient 02 PB and BM plasma, Th1 cytokines were detected immediately post-adoptive transfer (IFN-g=39 pg/ml, IL-2=8.6 pg/ml at peak); however these decreased to basal levels by day 3 (IFN-g=1.8 pg/ml) and day 2 (IL-2=2.6 pg/ml). The same trend in serum cytokines was observed for patient 01.
Patient 02 transduced LeY-T cells changed from a Th1 to a Th2-type polarization.
Patient 02 transduced LeY-T cells changed from a Th1 to a Th2-type polarization.
Therefore, our studies reveal that, after adoptive transfer, LeY CAR-T cells persist in vivo, maintain their expression of the CAR, but show rapid polarization from a Th1 to a Th2 phenotype.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.