Abstract
Abstract 4824
Recessive dystrophic epidermolysis bullosa (RDEB) is a severe inherited skin blistering disease caused by mutations in the type VII collagen (COL7A1) gene that encodes a major component in anchoring fibrils (Christiano et.al. Nat Gen 1993). Recently, iPSCs have been generated from RDEB patients, which provides promises on gene correction and future autologous Stem Cell Transplantation (SCT) (Itoh/Cairo/Christiano PNAS 2011). Currently, Allogeneic (Allo) SCT remains to be the best treatment for numerous malignant and non-malignant diseases in pediatric patients (Cairo et al BBMT 2008). This has also been supported by the first report on Allo-SCT in seven children with RDEB (Wagner et.al. N Engl J Med 2010). However no distinct anchoring fibrils were observed in the recipient skin. Moreover the functional cell populations are to be characterized. Stem cell populations with pluripotent properties, including unrestricted somatic stem cells (USSCs) have been isolated from HUCB, and may represent novel stem cells for the regenerative therapy for RDEB either by themselves, or by co-administration with BM or HUCB (Liao/Cairo et al, Exp. Hem, 2011).
Determine the potential of USSCs in promoting wound healing and treating RDEB.
USSCs were isolated from HUCB in the presence of 30% FBS and 10−7M dexamethasone, and characterized by RT-PCR, bisulfate sequencing and immunocytochemistry. A 1cm2 full-thickness excisional wound was created at the dorsal of NSG mice, followed by intradermal injection of 1 million USSCs or PBS at a 1cm- distance from the margin of the created wound. The wound areas were photographed and quantitated using ImageJ. Skin biopsies were taken at selected days and characterized by H&E staining and immunohistochemistry.
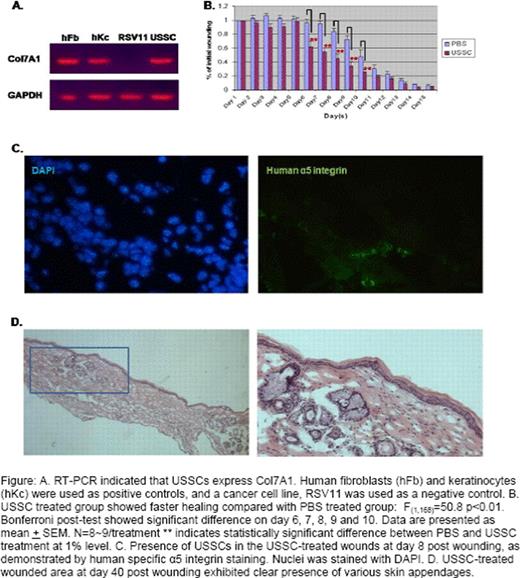
USSCs transcribe a low level of Nanog, Oct4 and Sox2, as compared to ES cells. Their Nanog and Oct4 expression is about 20- and 400- fold higher than that in human fibroblasts, and could be further activated at least 10 fold by treatment of DNA methylation inhibitor, 5-azacytidine. Their level of DNA methylation at the regulatory regions of Oct4 and Nanog genes is also intermediate between ES and fibroblasts. This suggests that USSCs represent a cell type that is developmentally an intermediate between ES cells and somatic cells. In addition, flow cytometry analysis indicated that USSCs also express stem cell surface antigen SSEA4. USSCs express Col7a1, the protein that is missing in the RDEB patients, at a level that is comparable to human keratinocytes and fibroblasts (fig A). This implies a potential ability of USSCs to rescue the RDEB defect by secreting COL7a1. In the mouse wounding model, USSC-treated wounds closed at a faster rate and Bonferroni post-test showed a significant difference between USSC and PBS treatment on days 6– 10 (Fig B). Immunological staining of tissue sections for endothelial protein CD31 showed increased vasculature in USSC-treated wounds at as early as three days post wounding, compared with PBS-treated wounds. Using immunostaining against human specific α5 and/or β1 integrin that is expressed specifically in USSCs, we have detected the presence of human cells within the wounded, but not unwounded basal layer of the skin, up to 1 month post wounding (Fig C). This indicates specific migration of USSCs to the wounded area and such a directed migration is likely to be mediated by the chemotactic interaction between stroma-derived factor 1(SDF-1) and CXCR4 that is expressed at the surface of USSCs. H&E staining on the skin biopsies taken at different time points indicated that USSCs promoted epithelialization and facilitated the formation and remodeling of epidermis. The resultant USSC-treated skin was less fibrotic, and more importantly, demonstrated formation of normal skin appendages in the center of the wounded area (Fig D). However, human cells were not detected in the skin appendages by immunological analysis, suggesting that accelerated epithelialization in the wound following USSC treatment has created an optimal environment for the migration of follicle progenitor cells from the unwounded skin to the center of the wound.
This study demonstrated the beneficial effect of HUCB derived USSCs in cutaneous regeneration and wound healing in a mouse wounding model. The ability of USSCs in secreting Col7A1 and promoting wound healing suggests their potential therapeutic application in treating RDEB.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.