Abstract
Abstract 772
Aptamers, which belong to a class of small molecule ligands composed of short single-stranded oligonucleotides, have emerged as probes over the last several decades; however, their potential clinical value has not yet been fully explored. Aptamers are developed from RNA/ssDNA libraries via a defined experimental process called Systematic Evolution of Ligands by EXponential enrichment. Synthetic aptamers are able to specifically bind an extremely wide variety of targets, including small molecules (dyes, metal ions, amino acids, and short peptides), biomacromolecules (nucleic acids and proteins), molecular complexes, viruses, and even live cells. Theoretically, the low nanomolar binding affinities and exquisite specificity of aptamers for their targets make them a versatile tool for disease diagnosis and targeting therapy. Compared to protein antibodies, as a small molecule probe aptamers are easily generated through chemical synthesis and simply modified with a variety of functional groups and/or tracking/imaging reporters. Notably, oligonucleotide aptamers have little or no immunogenicity or toxicity for in vivo use.
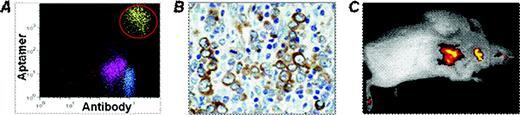
To develop potential clinical applications, a RNA-based aptamer specific for CD30 proteins was synthesized and conjugated with fluorochrome Cy5.5 as a reporter. Freshly cultured CD30-positive lymphoma cells were stained with the aptamer or anti-CD30 antibody as a standard control. Flow cytometry analysis showed that the aptamer probe is able to selectively bind to and detect lymphoma cells that express CD30. Specific cell binding of aptamer probes was also confirmed by fluorescence microscopy at a final concentration as low as 0.3 nM. Multi-color flow cytometry demonstrated that comprehensive immunophenotyping of lymphoma cells could be performed by using oligonucleotide aptamer probe(s) in combination with different antibodies (Figure A). Subsequently, we validated the use of synthetic aptamer for immunohistochemical study of formalin-fixed and paraffin-embedded tumor tissues including anaplastic large cell lymphoma and classical Hodgkin lymphoma. For this purpose, the aptamer was biotinylated and a horseradish peroxidase (HRP)-conjugated streptavidin system was employed for visualization of immunohistochemical staining. Tumor tissue studies revealed that the aptamer probes specifically recognized and selectively immunostained CD30-positive tumor cells of classical Hodgkin lymphoma and anaplastic large cell lymphoma, but did not react with background cells (Figure B). The aptamer probe optimally immunostained lymphoma cells with lower temperature antigen retrieval and shorter probing reaction times than typical antibody immunohistochemical protocols. In necrotic tumor tissues, the aptamer probe showed no non-specific background staining of cell debris although antibody-medicated immunohistochemical staining often does.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.