Abstract
As a result of previous transfusions, β-thalassemia major (TM) patients who have undergone curative hematopoietic stem cell transplantation (HSCT) are at increased risk of iron overload. There are, however, limited data on iron removal in such patients with either phlebotomy (PHL) or iron chelation. The aim of this study was to compare the efficacy, safety and convenience of the oral iron chelator deferasirox (DFX; Exjade®) with PHL for the treatment of iron overload in children with TM following HSCT, over a 1-year period.
LB03T is a prospective, randomized trial enrolling children with TM aged 2-<18 yrs who had undergone HSCT. Patients were chelation-naïve, hepatitis B- and C-negative, with confirmed iron overload (serum ferritin ≥500 ng/mL on ≥2 monthly occasions, and liver iron concentration [LIC] >3 mg Fe/g dry weight [dw]). Eligible patients were randomized to PHL (6 mL/kg blood/2 weeks) or DFX (10 mg/kg/day starting dose; 5 mg adjustments up to 20 mg/kg/day were permitted). One of the primary endpoints was change in LIC assessed using magnetic resonance imaging techniques. Changes in serum ferritin levels, hemoglobin (Hb), total iron binding capacity (TIBC), non-transferrin-bound iron (NTBI), adverse events (AEs) and compliance with study treatment (PHL: ratio of performed:planned; DFX: tablet count) were also assessed. Convenience of treatment was evaluated using parents' responses to pre-prepared questions.
27 patients were randomized to DFX or PHL; one patient randomized to PHL refused treatment, hence 12 patients received DFX and 14 received PHL. Mean age was 12.4 yrs and 53.8% were male. Patients were followed up for a mean of 12.0 months. 2 and 5 patients had DFX dose increases to 15 and 20 mg/kg/day, respectively. Mean DFX dose at last visit was 11.0 and 18.1 ng/mL in the LIC≤7 and LIC>7 groups, respectively.
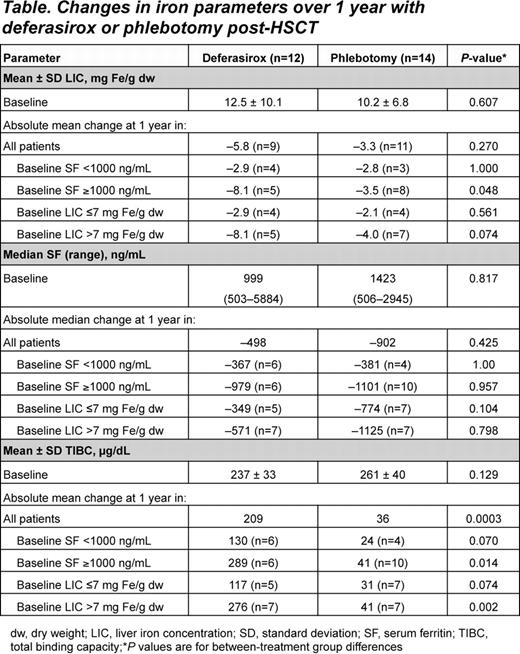
Median serum ferritin was significantly reduced from baseline over 12 months with DFX (–497.5 ng/mL, P=0.004 vs baseline) and PHL (–901.8 ng/mL, P<0.0001 vs baseline); there was no significant difference between groups (P=0.425). Mean LIC (for 20 patients with evaluable LIC following 1 yr of treatment) was significantly reduced with DFX (–5.78 mg Fe/g dw, P=0.0005 vs baseline) and PHL (–3.27 mg Fe/g dw, P=0.050 vs baseline); no significant difference between groups (P=0.270).
In patients with serum ferritin levels ≥1000 ng/mL at baseline, DFX resulted in a significantly greater decrease in mean LIC than PHL (–8.1 vs –3.5 mg Fe/g dw, P=0.048; Table). For TIBC, the increase with DFX was significantly greater than with PHL (P=0.0003). NTBI decreased significantly from baseline by –1.91 μmol/L (P=0.014; n=9) with DFX (baseline 2.0 μmol/L) and –2.83 μmol/L (P=0.0015; n=11) with PHL (baseline 2.3 μmol/L); there was no significant difference between groups (P=0.362). NTBI and LIC were positively correlated (R=0.565; P=0.0026) at baseline and at last follow-up (R=0.881; P<0.0001). Baseline mean Hb was 12.5 and 12.6 g/dL in the DFX and PHL groups, respectively; levels were maintained with DFX (change –0.17 g/dL; P=0.426) (PHL: change –0.53 g/dL; P=0.033); no significant difference between groups (P=0.279).
AEs reported for patients receiving DFX were skin rash [n=1], gastrointestinal upset [n=1], increased liver function tests [n=1]; for patients receiving PHL, difficulty with venous access [n=4] and distress during procedure [n=1] were reported. Compliance was excellent for 11 (91.7%) and 12 (85.7%); good for 1 (8.3%) and 1 (7.1%); and poor with 0 and 1 (7.1%) patients receiving DFX or PHL, respectively. Parents of 13/14 children randomized to PHL desired their children to receive DFX due to pain, risk of anemia and longer/more frequent hospital visits associated with PHL. Parents of 1/14 children were satisfied with PHL due to concerns over possible AEs with DFX.
In pediatric post-HSCT patients with TM, both LIC and serum ferritin were reduced with DFX and PHL over 1 year. In patients with higher baseline iron burden, DFX decreased LIC to a greater extent than PHL. TIBC was also significantly increased with DFX compared with PHL. DFX dose adjustments from 10 to 20 mg/kg/day were required to achieve therapeutic goals in some patients, underscoring the need for appropriate and timely dose adjustments. DFX had a clinically manageable safety profile, compliance was high and the majority of parents with children receiving PHL stated a desire to switch to DFX.
Inati:Novartis: Honoraria, Research Funding, Speakers Bureau. Cappellini:Novartis: Speakers Bureau. Taher:Novartis: Honoraria, Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.