Abstract
Abstract 97
There is no consensus or standard of care for front-line therapy for FL as demonstrated by our initial publication of the National LymphoCare Study (NLCS) showing that strategies used in newly diagnosed FL patients (pts) in the US included: watchful waiting (17.7%), rituximab (R) with chemotherapy (51.9%), R alone (13.9%), radiation therapy (5.6%), chemotherapy (3.2%), or a clinical trial (6.1%). Given the lack of observational data comparing the effectiveness of front-line R-chemotherapy regimens, we examined the outcomes for patients with stage III/IV FL receiving R with cyclophosphamide, vincristine, and prednisone (RCVP), R with cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP), or R with a fludarabine-based regimen (RFlu) as first-line therapy.
The NLCS is a prospective, multicenter, observational study collecting data on 2,738 previously untreated patients (pts) with FL diagnosed from 2004 to 2007 at 265 sites in the US. Initial management decisions were made by the treating physician without protocol-specified treatment assignments. Descriptive statistics for the baseline characteristics of pts who received RCVP, RCHOP, and RFlu were compared using Pearson Chi-square tests. A generalized logistic model was used to identify baseline factors of clinical interest (FLIPI, sex, practice setting [academic vs. community]) that were correlated with treatment. Kaplan–Meier estimation was used to evaluate progression-free survival (PFS) and overall survival (OS) for the treatment regimens; comparisons for the 2-year landmark estimates were obtained using a Wald statistic. PFS was defined as the number of days from diagnosis up to and including the date of progressive disease (PD) as assessed by the treating physician or death from any cause. Pts who had not yet experienced PD at the time of analysis were censored at the date of the most recent response assessment. To evaluate the effects of treatment on PFS and OS, Cox proportional hazards models were used controlling for FLIPI, sex, community or academic practice setting, and maintenance R or observation following treatment.
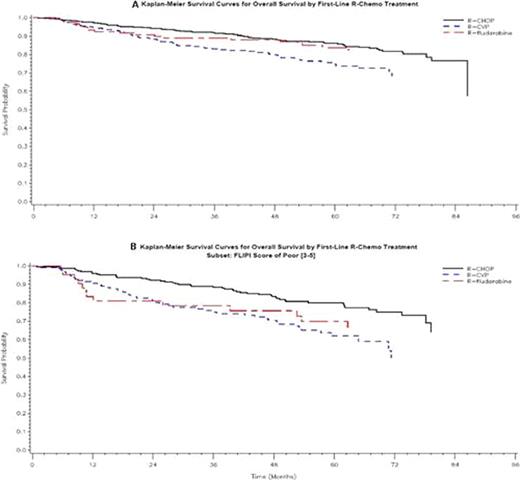
Of the 2,738 pts enrolled in NLCS, 926 pts were identified as having stage III/IV FL and having received the regimens of interest. Within this cohort 60% received RCHOP (n=558), 27% received RCVP (n=247) and the remaining 13% received RFlu (n=121). Baseline characteristics were correlated with induction treatment and whether a patient received subsequent R maintenance. Women (odds ratio [OR]=1.50, 95%CI 1.05–2.13) and pts aged >60 years (OR=2.36, 95%CI 1.64–3.38) more commonly received RCVP than RCHOP, and pts with LDH > upper limit of normal (OR=1.75, 95%CI 1.02–3.03) more commonly received RCHOP than RFlu. A greater % of pts receiving RCVP (61%) had poor-risk FLIPI (3–5) vs. RCHOP (49%) or RFlu (44%). In this cohort, 60% of RCVP, 43% of RCHOP, and 46% of RFlu pts received maintenance R. The overall response rates for RCVP, RCHOP, and RFlu were 88%, 94%, and 96%, respectively, and 2-year PFS was 72% (95% CI 66–78%) for RCVP, 78% (95%CI 74–81%) for RCHOP, and 76% (95%CI 67–-83%) for RFlu. There were no significant differences in PFS between treatments after adjustment (p= 0.07). However, with a median follow-up of 58 months there were significant differences in unadjusted OS (p=0.03), with 2-year OS probabilities of 88% (95%CI 84–92%) for RCVP, and 91% (84–95%) for RFlu, and 94% (92–96%) for RCHOP (Figure A). After adjustment for sex, FLIPI, practice setting, and maintenance use, the OS benefits of RCHOP persisted over RCVP HR=1.67 (95%CI 1.15–2.42) but not RFlu HR=0.94 (0.53–1.68). The benefits of RCHOP were even more pronounced in poor risk FLIPI pts (Figure B).
First-line R-chemotherapy regimens in clinical practice have high overall response rates and high 2-year PFS and OS. With 4.75 years of follow-up in this large pt cohort, these regimens appear similar in response rates and early PFS and OS with suggestions that RCHOP may provide benefits over RCVP particularly for poor risk patients.
Sinha:Celgene: Research Funding. Friedberg:Genentech: Consultancy; astellas: ; Lilly:; Abbott/Trubion:; Seattle Genetics: Honoraria; Cephalon: Consultancy. Hirata:Roche: Equity Ownership; Genentech Inc/Roche: Employment. Flowers:Seattle Genetics: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech/Roche (unpaid): Consultancy; Novartis: Research Funding; Spectrum: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.