In this issue of Blood, Zhang at al report that hypomorphic mutations in FHL-causing genes correlate with a later-onset and a more indolent course in adult patients.1 Another important study on the same disease led to the identification of alterations in an evolutionarily conserved intronic region, thus highlighting the importance of searching for aberrations outside the coding region of the implicated gene.2
Familial HLH is well recognized in children but rarely diagnosed in adults. Moreover, even though alterations of at least 7 genes are associated with the disease, in the majority of patients the molecular defect cannot be found.
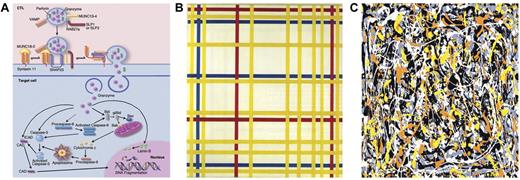
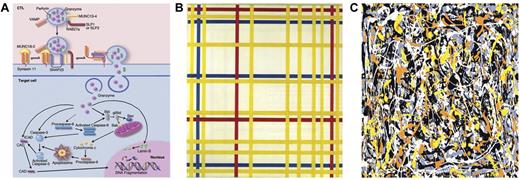
Cytolysis: a Mondrian linearity or Pollock complexity. (A) Cytolytic pathways. Professional illustration by Marie Dauenheimer. (B) Broadway Boogie-Woogie by Piet Mondrian. (C) Untitled No. 3 by Jackson Pollock.
Cytolysis: a Mondrian linearity or Pollock complexity. (A) Cytolytic pathways. Professional illustration by Marie Dauenheimer. (B) Broadway Boogie-Woogie by Piet Mondrian. (C) Untitled No. 3 by Jackson Pollock.
The huge number of congenital disorders of the immune system discovered in the past 20 years has greatly contributed to the understanding of the physiology of the immune response.3 Remarkably, the easy availability of the “sick cell” gave the unique opportunity to search for an intimate link among clinical phenotype, genetic alteration, and the pathogenic mechanism of that individual immunologic functional abnormality. The discovery of genetic alterations in so many molecules led to an unexpected dissection of the numerous functional and biochemical pathways into distinct molecular events, thus favoring a detailed understanding of the role of that specific molecule. What important information came out of the discovery of genetic alterations of the common γ chain besides its pathogenic role in the SCID-X1? Gamma chain and T-cell development, the role of several γ-related cytokines in T-cell differentiation and functions, molecular therapy and immune reconstitution through gene transduction, and the general role of the molecule in cell-cycle progression and tumorigenesis are only a few examples that highlight the importance of studies of primary immunodeficiencies (PIDs) that go far beyond the understanding of the cause of a disease.4,5 Very often, studies in this field highlight shared aspects, which also help unravel complex issues in other fields of modern medicine.
It is not surprising that the greater the number of the distinct immunologic disorders, the greater the diversity of presenting signs. Much time elapsed from the paradigm that a severe PID should have been ruled out if the patient had recovered from an infection by the time expected. Thus, no one would have imagined 10 years ago that the onset of a severe PID could be during adulthood. Actually, an eminent physician scientist in the field, Jean-Laurent Casanova, in a lecture at the European Society of Immunodeficiencies, argued that every time the clinical course of an infection is life threatening there is an underlying immunodeficiency to discover. Although this opinion may be, to some extent, extreme, if it will be proven true in the future, it certainly implies that the true prevalence of congenital immunodeficiencies will not be defined until all patients with very severe infectious diseases have been investigated for an underlying immunologic disorder.
The interesting study by Zhang et al here from the Filipovich group reports that hypomorphic mutations in FHL-causing genes correlate with the later-onset disease,1 as previously reported in this journal in one family.6 This genetic alteration is associated with a more indolent course, resulting in adult presentation with a few cases even in their seventies. This study also points out that it is quite difficult to define a boundary between causative genetic alterations and a genetic variation that predisposes to the disease. In this study, the A91V-PRF1 genotype was found in half of adult patients with FHL in both the heterozygous and homozygous states. This variation has previously been found in the general population, with its prevalence ranging between 4% and 7%. However, the prevalence of the variation in FHL patients is much higher, up to 26% in a cohort of well-characterized patients.7 Taken together these data imply that this variation is an important genetic susceptibility factor. A number of studies also document that the A91V substitution is not clinically neutral,8 even though it may variably affect cytotoxic activity.9,10
Unfortunately, the more complete the understanding of the disease pathogenesis, the more complex the diagnosis becomes. At the very beginning, when it became clear that most of the defects of cytotoxic activities were related to alterations of molecules implicated in the cytolytic signaling pathway (see figure panel A), physician-scientists thought of the disease pathogenesis in too simplistic a way. At least 7 genes (PRF1, MUNC13-4, STX11, RAB27A, STXBP2, SH2D1A, and BIRC4) are known to be associated with FHL, but unfortunately, in the majority of patients with a well-established clinical and functional diagnosis of HLH, molecular diagnosis is still not possible. Paralleling contemporary art masterpieces, many of us thought at the very beginning of Mondrian linearity (figure panel B). Indeed, the complexity of the relationship among clinical phenotypes, altered biochemical pathways, and individual gene alteration is rather comparable with Jackson Pollock complexity (figure panel C), which seems to clearly depict the cross-interference between distinct pathways, the huge number of molecules in that specific pathway, the number of distinct genetic and environmental predisposing factors, and the functional role of distinct molecular alterations of that molecule. It should also be kept in mind that many viruses may per se alter cell physiology in the absence of any genetic disorder.11 Another study in this issue of Blood by Meeths et al on the same disease highlights the importance of also searching for aberrations outside the coding region of UNC13D gene in patients with FHL.2 This study addresses the important issue of defining a pathogenic mechanism in FLH patients with no molecular diagnosis. The alteration was located in an evolutionarily conserved intronic region and was consistent with a loss-of-function mutation in that it resulted in defective natural killer cell degranulation and cytotoxicity, as well as absent MUNC13-4 expression. The conclusion reached by these authors is that the search for alterations outside the coding regions helps in the diagnosis as well as in deciding on therapy for such patients. Furthermore, the strategy of sequencing of evolutionarily conserved noncoding regions should be applied to the diagnostics of the whole field of primary immunodeficiencies. In a number of cases of PIDs, despite a clear clinical and functional phenotype suggestive of a specific diagnosis, no molecular confirmation can be reached leading to the hypothesis that a novel altered gene might be responsible. Although this may be true in many cases, the possibility of a genetic alteration of a noncoding region should be taken in consideration.
A further implication of these observations is that the dream of screening through microarray technologies all the genes coding for molecules in a function-related pathway is likely to remain a dream because of the importance of the noncoding regions. A further point of interest in the study by Meeths et al is the observation of a 9-fold reduction in the transcription of the mutated UNC13D allele relative to the wild-type in lymphocytes. The relative frequency of the transcript from the mutated allele in CD4+, CD8+, and CD56+ cells was significantly less than that observed in CD14+ cells, suggesting a role for the intronic mutation in cell-specific transcription of the UNC13D gene. One could argue that in the future the search for protein expression or gene transcription has to be performed only in the presumed sick cell to be informative. In light of these considerations, going back to the functional assessment as the key point of the diagnostic procedure for PIDs might prove to be the more appropriate strategy.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■