Abstract
Donor T cells contribute to the success of allogeneic hematopoietic stem cell transplantation (alloSCT). Alloreactive donor T cells attack leukemia cells, mediating the GVL effect. Donor T cells, including the memory T cells (TM) that are generated after infection, also promote immune reconstitution. Nonetheless, leukemia relapse and infection are major sources of treatment failure. Efforts to augment GVL and immune reconstitution have been limited by GVHD, the attack by donor T cells on host tissues. One approach to augmenting GVL has been to infuse ex vivo–generated T cells with defined specificities; however, this requires expertise that is not widely available. In the present study, we tested an alternative approach, adoptive immunotherapy with CD8+ TM from donors vaccinated against a single minor histocompatibility antigen (miHA) expressed by leukemia cells. Vaccination against the miHA H60 greatly augmented TM-mediated GVL against mouse chronic-phase (CP-CML) and blast crisis chronic myeloid leukemia (BC-CML). TM-mediated GVL was antigen specific and was optimal when H60 expression was hematopoietically restricted. Even when H60 was ubiquitous, donor H60 vaccination had a minimal impact on GVHD. TM from lymphocytic choriomeningitis virus (LCMV)–immune and H60-vaccinated donors augmented GVL and protected recipients from LCMV. These data establish a strategy for augmenting GVL and immune reconstitution without elaborate T-cell manipulation.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) can be a curative therapy for patients with hematologic malignancies. Mature donor T cells in the allograft are critical for reconstituting T-cell immunity in recipients and mediate an alloimmune antileukemia/lymphoma effect called GVL. In MHC-matched alloSCT, alloimmune T cells target minor histocompatibility antigens (miHAs), which are peptide products of polymorphic genes that distinguish hosts from donors.1 Unfortunately, alloreactive T cells also attack normal host tissues, causing GVHD. A longstanding and elusive goal in the alloSCT field has been to develop approaches that preserve GVL and immune reconstitution while minimizing GVHD. A second challenge has been how to augment GVL to overcome GVL resistance, as cancer relapse is the single greatest cause of death after transplantation.2 The risk of relapse is not spread evenly across cancer types. Some malignancies, such as chronic-phase chronic myelogenous leukemia (CP-CML), are extremely GVL sensitive,3 whereas other types of leukemias, such as blast-crisis CML (BC-CML) and acute myelogenous leukemia, are relatively GVL-resistant.3,4
One approach for improving GVL would be to broadly augment the alloimmune response by reducing the intensity of immunosuppression. However, this increases the risk for serious and life-threatening GVHD.5 Another strategy has been to develop T-cell therapies with a single target specificity, such as those that recognize a single miHA, with the idea that such T cells could augment GVL with a reduced risk for inducing GVHD.1,6 These T cells can be clonal, polyclonal, or genetically modified to express defined antigen receptors.7,8 Although there are some promising results,9,10 such approaches have not yet been widely applied. Transferred cells have in general not survived well in vivo, perhaps because they were activated effectors. In addition, these methodologies are labor intensive and require technical expertise and facilities not available at most centers.
We reasoned that an alternative to T-cell engineering would be to increase the frequency of allograft T cells that mediate GVL by vaccinating donors against a single miHA. There is evidence that functional T-cell memory against miHAs can be generated in vivo in humans.11-13 GVHD is increased when donors are multiparous,12 presumably because of priming against fetal miHAs. Blood transfusion of even aplastic anemia patients increases the risk of HLA-matched allograft rejection.13 If donors could be vaccinated against single miHAs, then the selective transfer of donor memory T cells (TM) could improve GVL. TM generated in the donor by prior pathogen infection would also be transferred, thereby augmenting immune reconstitution.
In the present study, we show that miHA vaccination of donors greatly increases CD8+ TM-mediated GVL against GVL-sensitive mouse CP-CML (mCP-CML)14 and GVL-resistant mouse BC-CML (mBC-CML)15 induced by bcr-abl or bcr-abl and NUP98/HOXA9 fusion cDNAs, respectively. MiHA-reactive TM underwent dramatic expansion in the host, independent of CD4 help, which is consistent with their memory phenotype. GVL was antigen specific and required the target antigen to be limited to the host's hematopoietic system. Even when the target antigen was ubiquitously expressed in the host, donor miHA vaccination had little effect on GVHD. The transfer of CD8+ TM from lymphocytic choriomeningitis virus (LCMV)–immune donors who were also vaccinated against an miHA both improved GVL and protected recipients from LCMV infection. These data establish a relatively simple approach for augmenting GVL that could be applied in the clinical setting because it does not require the facilities and technical expertise necessary to create T-cell lines and gene-modified T cells.
Methods
Mice
B6.H60 and actH60 mice were obtained from D.R. and bred at Yale University (New Haven, CT). C3H.SW mice were purchased from The Jackson Laboratory and bred at Yale University. DEC205−/− mice were obtained from Michel Nussenzweig (Rockefeller University, New York, NY). C57BL/6 (B6) mice were purchased from Taconic/National Cancer Institute.
Cell separations
Donor BM in all experiments was depleted of T cells using anti-Thy1.2 microbeads and the AutoMACS (Miltenyi Biotec) as described previously.16 In some experiments, bead-purified CD8+ T cells were stained with anti-CD62L (Mel-14; BD Pharmingen) and anti-CD44 (IM7; BD Pharmingen) and sorted into CD62L+CD44− naive and CD44+ memory populations using a FACSAria cell sorter (BD Biosciences). CD8 cells were purified from spleen and lymph nodes by positive selection using biotin-conjugated Abs against CD8 (clone TIB105; laboratory-prepared), followed by streptavidin-conjugated magnetic beads (Miltenyi Biotec) and separation on an AutoMACS (Miltenyi Biotec). CD8 cells were > 90% pure, with a CD4 T-cell contamination of < 0.2%. For further separations of naive I cells (TN) and TM, enriched CD8 cells were incubated with anti–CD8-PE (53-7.6; BD Pharmingen), anti–CD62L-FITC (Mel-14; BD Pharmingen), and anti-CD44-allophycocyanin (Pgp-1; BD Pharmingen). Cells were sorted into CD8+CD62L+CD44− TN and CD8+ CD44+ TM using a FACSAria cell sorter (BD Biosciences).
DEC-H60 construction, production, and vaccination
To generate the recombinant anti-DEC205 Ab modified to express the sequence for LTFNYRNL (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), the parent heavy-chain vector was cut with NotI and NheI to excise the sequence encoding OVA. This was ligated with a synthesized double-stranded sequence encoding LTFNYRNL and 15 additional amino acids that flank both the amino and carboxy ends. NotI and NheI sites were also included. The construct was confirmed by DNA sequencing. Ab was produced by transfecting 293T cells with light-chain and H60-modified heavy chain. Supernatant was harvested 6 days later and purified over a protein G column (HiTrap; GE Life Sciences). FGK4517 was laboratory-grown and purified. To generate H60-reactive CD8+ TM, mice were immunized by IV injection with 50μg of DEC-H60 and IP injection of 50 μg of FGK45. TM were harvested from mice at least 90 days after vaccination.
Tetramers
The H-2Kb/ LTFNYRNL tetramer conjugated to allophycocyanin was laboratory-prepared as described previously.18
Flow cytometry
The following Abs were used to analyze/sort memory T cells: anti–CD8-PE (53-7.6; BD Pharmingen), anti–CD62L-Pacific Blue (Mel14; BioLegend), anti–CD44-FITC (Pgp-1; BD Pharmingen), and anti–CD127-PerCP (SB/199; BioLegend). For the intracellular cytokine staining, 5 × 106 splenocytes or lymph node cells (interscapular, axillary, cervical, mesenteric, and inguinal) were harvested and cultured with LTFNYRNL peptide (1.0μM; Keck Laboratory, Yale University School of Medicine) at 37°C for 6 hours; GolgiPlug (BD Biosciences) was added at the same time. Before permeabilization, cells were incubated with ethidium monoazide to allow exclusion of dead cells. Cells were stained with Abs against CD8 (PE; BD Biosciences) and CD44 (FITC; BD Biosciences), permeabilized, and then stained with Abs against IFN-γ (allophycocyanin clone XMG1.2; BD Biosciences) or isotype controls (for the anticytokine Abs). For flow cytometric analysis of leukemic cells in peripheral blood, whole blood was stained with Abs against Gr-1 (FITC clone RB6-8C5; BD Pharmingen) and nerve growth factor receptor (NGFR; Alexa Fluor 647 clone 20.4; laboratory-prepared). Results were analyzed with FlowJo Version 9 software (TreeStar).
Retrovirus production
MSCV2.2 expressing the human bcr-abl p210 cDNA and a nonsignaling truncated form of the human low-affinity NGFR driven by an internal ribosome entry site (M-p210/NGFR) was as described previously.14 MSCV2.2 expressing the NUP98/HOXA9 and EGFP downstream of the internal ribosome entry site (M-NH/EGFP) was a gift from D. G. Gilliland19 (Merck Research Laboratories). Retroviral supernatants were generated as described previously.14,20
mCP-CML and mBC-CML generation
p210-infected progenitors were generated as described previously.14 To generate mBC-CML, mice were injected on day −6 with 5 mg of 5-fluorouracil (Pharmacia & Upjohn). On day −2, BM cells were harvested and cultured in prestimulation medium (DMEM, 15% FBS, 6 ng/mL of IL-3, 10 ng/mL of IL-6, and 10 ng/mL of SCF; all cytokines were from Peprotech). On days −1 and 0, cells were resuspended at 2 × 106/mL in prestimulation medium with the addition of the retroviral supernatants Polybrene (4 μg/mL; Sigma-Aldrich) and HEPES (100mM).14 After this, 7.5 × 105 cells that underwent spin infection were injected into 600 cGy sublethally irradiated B6 hosts. Premorbid mice were killed and splenocytes were frozen. These cells were passaged in sublethally irradiated mice, from which splenocytes were harvested and frozen (secondary mice). mBC-CML cells were then cloned by injecting 1000 live EGFP+ cells into sublethally irradiated hosts, which results in end-stage leukemia in 40-60 days. Individual spleens were frozen in experiment-sized aliquots. Expression of H60 on H60+ mBC-CML cells was confirmed by flow cytometry (clone 205326; R&D Systems).
BM transplantations for GVL experiments
All transplantations were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee. On day 0, B6.H60 hosts were irradiated (450 cGy × 2) and reconstituted with 5 × 106 T cell–depleted C3H.SW BM with 104 mBC-CML cells, 7 × 105 BM cells that had undergone spin infection with M-p210/NGFR, and T cells as specified in the text and figure legends. Mice were bled weekly beginning on day 7. Death was considered to be from leukemia based on the presence of leukemia cells in the peripheral blood before death and spleen weight at necropsy.
GVHD-inducing transplantation and analysis
To create B6.H60→act.H60 chimeras, actH60 mice were irradiated (500 cGy × 2) and reconstituted with 5 × 106 B6.H60 BM cells. Chimeras were rested 2-3 months before a second GVHD-inducing transplantation. Chimeras were reirradiated (450 cGy × 2) and reconstituted with 7 × 106 T cell–depleted C3H.SW BM with no T cells or with C3H.SW CD8+ TN or TM or TM from DEC-H60–vaccinated donors. Mice were weighed and scored for GVHD 2-3 times a week. Weights from mice that died or were killed were included in averages for subsequent time points at the last value recorded. Cutaneous GVHD was assessed as described previously.21 Mice were killed 45 days after transplantation to harvest tissues for histopathologic analysis. Slides were scored by pathologists who are experts in skin (J.M.) and gastrointestinal disease (A.J.D.) who did not have knowledge as to experimental group, as described previously.22,23
LCMV infection and virus titration
To generate LCMV-specific CD8+ TM, C3H.SW mice were infected IP with 2 × 105 PFU of LCMV-Armstrong; 2 × 106 PFU of LCMV-clone 13 was used to challenge mice intravenously after allogeneic BM transplantation. Infectious LCMV was quantitated by a plaque assay, as described previously.24
Results
Model system
To study miHA vaccination, we needed a system in which we could track miHA-reactive CD8 cells, recipient mice that express the antigen, and the ability to make antigen-expressing model leukemias. We chose as a model miHA, the Kb-restricted LTFNYRNL peptide derived from H60.25-27 MHCI tetramers and LTFNYRNL-induced IFN-γ expression allow H60-reactive CD8 cells to be tracked by flow cytometry. We (D.R.) created 2 types of mice that express H60. The first are congenic for H60 (B6.H60),28,29 the expression of which is mostly limited to hematopoietic cells.25 The second are transgenic for H60, driven by an actin promoter (actH60), wherein H60 is ubiquitously expressed, but at a lower level than in B6.H60 mice.29 To generate leukemias that do or do not express H60, we used oncogene retrovirus transduction of BM from B6.H60 or wt B6 mice, respectively. To create mCP-CML, we infected BM with retrovirus encoding bcr-abl linked by an internal ribosome entry site to a nonsignaling human NGFR cDNA (bcr-abl/NGFR).14 To create mBC-CML, we coinfected BM with bcr-abl-NGFR and a second retrovirus encoding the NUP98/HOXA9 fusion cDNA linked by an internal ribosome entry site to an EGFP cassette. mCP-CML is dominated by maturing myeloid cells with few myeloblasts and is very GVL sensitive,14 whereas mBC-CML is dominated by myeloblasts and is relatively GVL resistant.15 Finally, we needed a convenient method to create LTFNYRNL-reactive TM. We cloned the sequence for LTFNYRNL into a construct encoding the heavy chain for an Ab against mouse DEC20530 (supplemental Figure 1). We then used the resultant recombinant Ab (DEC-H60) and an agonist Ab against CD40 (FGK45)30 as an adjuvant to vaccinate donors.
DEC-H60 vaccination
DEC-H60 vaccination of B6 mice expanded CD8+ T cells that bound the LTFNYRNL-tetramer (TetH60+; supplemental Figure 2A). This expansion was H60-specific, as we were unable to expand TetH60+ cells in B6.H60 or actH60 controls, which presumably had already deleted LTFNYRNL-reactive T cells. Immunization mostly depended on the expression of DEC205, as we saw far fewer TetH60+ cells in vaccinated DEC205−/− mice31 (supplemental Figure 1A).
We next used DEC-H60 to create LTFNYRNL-reactive CD8 TM (TMH60) in C3H.SW mice (H-2b; H60−). Nearly 30% of blood and spleen CD8 cells were TetH60+ 7 days after immunization, and a similar percentage produced IFN-γ with LTFNYRNL stimulation (supplemental Figure 1B-D). As is typical during the generation of CD8+ TM, the frequency of TetH60+ cells in blood (supplemental Figure 1D) and spleen (not shown) declined over time. Initially, most TetH60+ cells were CD62L−; however, the frequency of CD62L+ cells progressively increased such that by 3 months after vaccination, > 60% of TetH60+ cells were CD44+CD62L+ central memory T cells (supplemental Figure 2D). By days 90-120 after vaccination, 4%-10% of CD8+CD44+ cells in blood or spleen were TetH60+ and the majority of these were CD44+CD62L+ (supplemental Figure 2E).
Expansion of miHA-reactive TM after transplantation
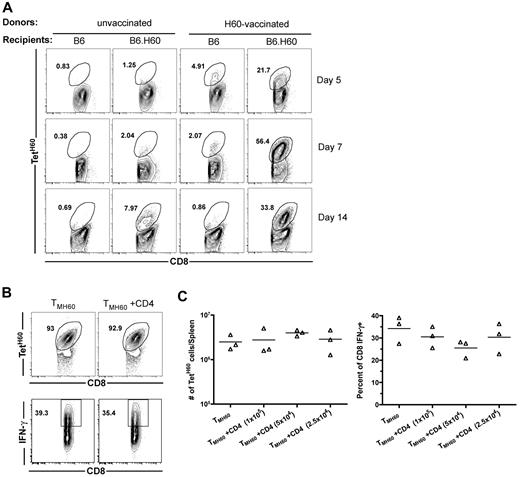
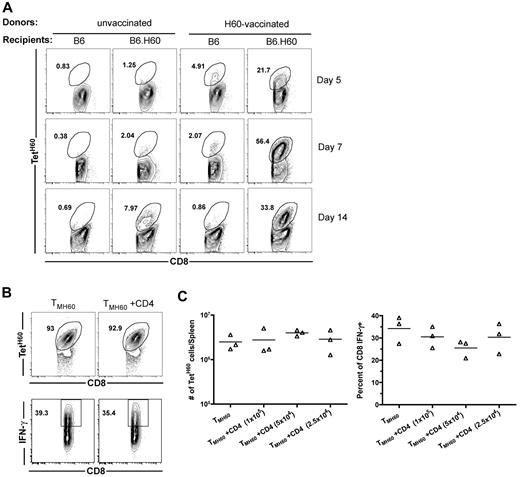
We next investigated the expansion of LTFNYRNL-reactive CD8 cells after transplantation of TM from H60-vaccinated donors. B6.H60 or control B6 (H60−) mice were irradiated and reconstituted with C3H.SW BM plus 106 total CD8 cells from H60-vaccinated or control unvaccinated C3H.SW mice. All mice also received 105 C3H.SW CD4 cells as primary anti-LTFNYRNL responses are Th-cell dependent (not shown). TetH60+ cells underwent dramatic and rapid expansion in B6.H60 recipients of TMH60, whereas the frequency of TetH60+ cells in transplanted B6 mice remained similar to that in the pretransplantation CD8 cells (Figure 1A), demonstrating that the expansion of TetH60+ cells is LTFNYRNL-dependent. In contrast, there were very few TetH60+ cells in B6.H60 recipients of CD8 cells from unvaccinated mice (Figure 1A).
H60-reactive TM expand after transplantation into H60+ recipients without CD4 help. (A) Expansion of LTFNYRNL-reactive CD8 cells requires the host to be H60+. B6.H60 or B6 (H60−) mice were irradiated and reconstituted with C3H.SW BM with 106 CD8 cells from unmanipulated or H60-vaccinated C3H.SW donors along with 105 CD4 cells from unmanipulated C3H.SW mice. (A) Representative flow cytometry. (B-C) B6.H60 mice were irradiated and reconstituted with C3H.SW BM with 2 × 105 C3H.SW TMH60 with or without graded numbers of CD4 cells from unmanipulated C3H.SW mice. (B) Representative flow cytometry of splenocytes at day 7 after transplantation, with gating on CD8+ cells. TetH60 binding and IFN-γ staining after LTFNYRNL stimulation are shown. (C) The total numbers of TetH60+ and percentages of IFN-γ+ CD8 cells are shown. P > .1 comparing TMH60 with TMH60 plus any CD4 dose. Data are representative of 2 independent experiments.
H60-reactive TM expand after transplantation into H60+ recipients without CD4 help. (A) Expansion of LTFNYRNL-reactive CD8 cells requires the host to be H60+. B6.H60 or B6 (H60−) mice were irradiated and reconstituted with C3H.SW BM with 106 CD8 cells from unmanipulated or H60-vaccinated C3H.SW donors along with 105 CD4 cells from unmanipulated C3H.SW mice. (A) Representative flow cytometry. (B-C) B6.H60 mice were irradiated and reconstituted with C3H.SW BM with 2 × 105 C3H.SW TMH60 with or without graded numbers of CD4 cells from unmanipulated C3H.SW mice. (B) Representative flow cytometry of splenocytes at day 7 after transplantation, with gating on CD8+ cells. TetH60 binding and IFN-γ staining after LTFNYRNL stimulation are shown. (C) The total numbers of TetH60+ and percentages of IFN-γ+ CD8 cells are shown. P > .1 comparing TMH60 with TMH60 plus any CD4 dose. Data are representative of 2 independent experiments.
Although CD4 cells are required for optimal anti-LTFNYRNL primary responses, we considered the possibility that recall responses by TMH60 may not require CD4 cells. B6.H60 mice were irradiated, and reconstituted C3H.SW BM and 2 × 105 sort-purified CD8+CD44+ cells from DEC-H60–vaccinated mice (5% TetH60+) with or without 105, 2.5 × 105, or 5 × 105 CD4 cells from unmanipulated C3H.SW mice. By day 7 after transplantation, LTFNYRNL-reactive CD8 cells from H60-vaccinated mice had undergone dramatic expansion (> 100-fold over the infused number in the spleen alone), with the majority of all CD8 cells being TetH60+ (Figure 1B representative flow cytometry; Figure 1C quantitation). Neither the frequency nor the total number of TetH60+ cells was affected by the addition of CD4 cells (Figure 1B-C). To evaluate whether the function of LTFNYRNL-reactive cells differed with or without CD4 cells, we LTFNYRNL-stimulated splenocytes from the same recipients and stained for intracellular IFN-γ. Approximately 35% of CD8 cells produced IFN-γ with or without additional CD4 cells (Figure 1B-C). Therefore, neither expansion nor effector maturation as measured by IFN-γ production required CD4 help. Expansion of SIINFEKL-reactive CD8+ TM (created by vaccination with an OVA-modified anti-DEC205) in similarly transplanted OVA-transgenic mice32 was also robust without CD4 help (data not shown).
TMH60 mediate potent GVL against mBC-CML
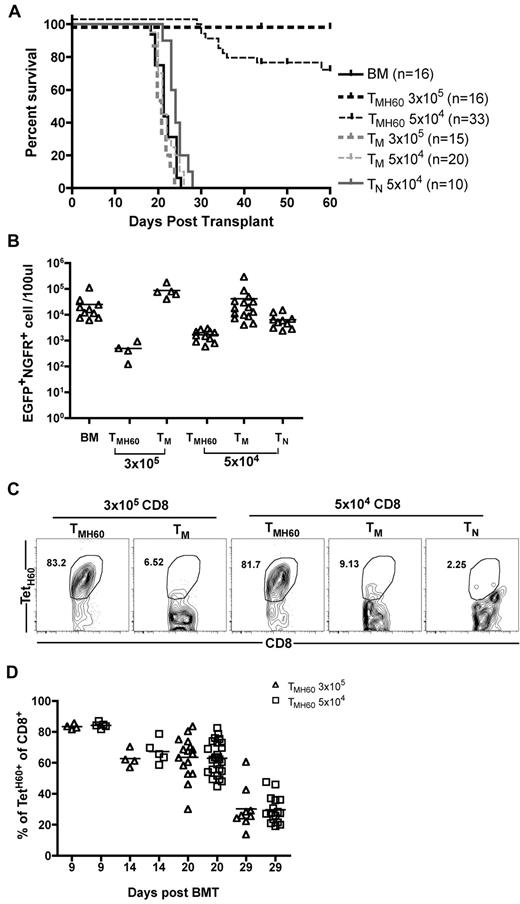
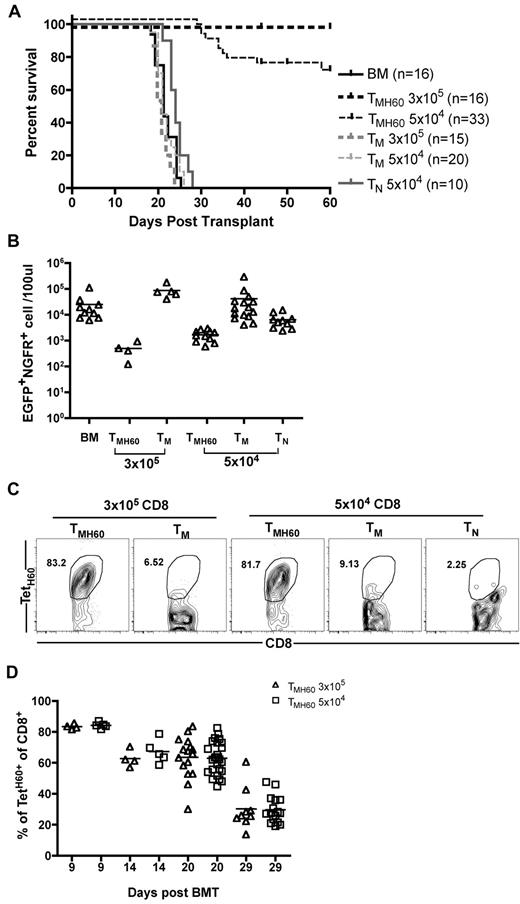
To determine whether DEC-H60 vaccination augments GVL mediated by donor CD8+ TM, B6.H60 mice were irradiated and reconstituted with C3H.SW BM and 104 B6.H60 mBC-CML cells with no T cells or with 3 × 105 or 5 × 104 CD8+ TM from DEC-H60-vaccinated C3H.SW mice (11% TetH60+). Additional controls received transplantations with C3H.SW BM and B6.H60 mBC-CML with 3 × 105 or 5 × 104 CD8+ TM or 5 × 104 CD8+ TN from unmanipulated C3H.SW mice. Neither TM nor TN from control C3H.SW mice prolonged survival compared with mice that received no T cells (Figure 2A-B). In contrast, CD8+ TM from H60-vaccinated mice, containing as few as 5100 TetH60+ cells, were potent mediators of GVL as measured by survival (Figure 2A) and the numbers of mBC-CML cells in peripheral blood (Figure 2B). Substantial anti-LTFNYRNL responses were only seen in the TMH60 recipients (Figure 2C-D).
TMH60 mediate GVL against mBC-CML. B6.H60 mice were irradiated and reconstituted with C3H.SW BM, B6.H60 mBC-CML cells with no T cells, or with 3 × 105 or 5 × 104 CD8+CD44+ cells from DEC-H60–vaccinated or unmanipulated C3H.SW mice or 5 × 104 CD8+CD44−CD62L+ TN from unmanipulated C3H.SW mice. (A) Survival. All deaths were from leukemia. P < .0001 comparing BM alone with any TMH60 group; P = .0124 comparing BM alone with TN recipients; P > .2 comparing BM alone with any TM group. P ≤ .026 comparing any TMH60 group with any TM or TN group. (B) Number of EGFP+NGFR+ cells in peripheral blood at day 14. P < .05 comparing either TMH60 recipient group with its TM control; P < .008 comparing any TMH60 recipient group with the BM alone control. TetH60+ cells accumulated only in TMH60 recipients. (C) Representative flow cytometry of peripheral blood at day 9. (D) Quantitation at days 9, 14, 20, and 29. Each symbol represents data from an individual animal; solid lines are mean values. Data are pooled from 3 independent experiments with similar results.
TMH60 mediate GVL against mBC-CML. B6.H60 mice were irradiated and reconstituted with C3H.SW BM, B6.H60 mBC-CML cells with no T cells, or with 3 × 105 or 5 × 104 CD8+CD44+ cells from DEC-H60–vaccinated or unmanipulated C3H.SW mice or 5 × 104 CD8+CD44−CD62L+ TN from unmanipulated C3H.SW mice. (A) Survival. All deaths were from leukemia. P < .0001 comparing BM alone with any TMH60 group; P = .0124 comparing BM alone with TN recipients; P > .2 comparing BM alone with any TM group. P ≤ .026 comparing any TMH60 group with any TM or TN group. (B) Number of EGFP+NGFR+ cells in peripheral blood at day 14. P < .05 comparing either TMH60 recipient group with its TM control; P < .008 comparing any TMH60 recipient group with the BM alone control. TetH60+ cells accumulated only in TMH60 recipients. (C) Representative flow cytometry of peripheral blood at day 9. (D) Quantitation at days 9, 14, 20, and 29. Each symbol represents data from an individual animal; solid lines are mean values. Data are pooled from 3 independent experiments with similar results.
TMH60 mediate potent GVL against mCP-CML
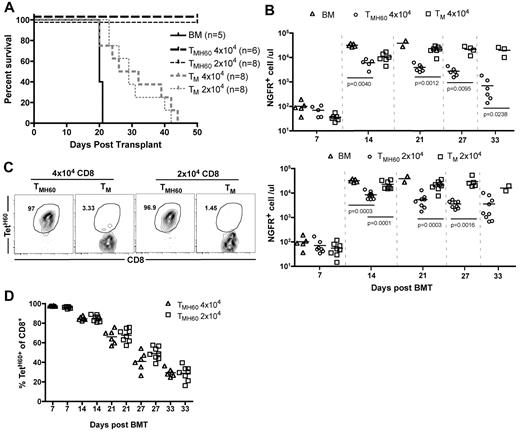
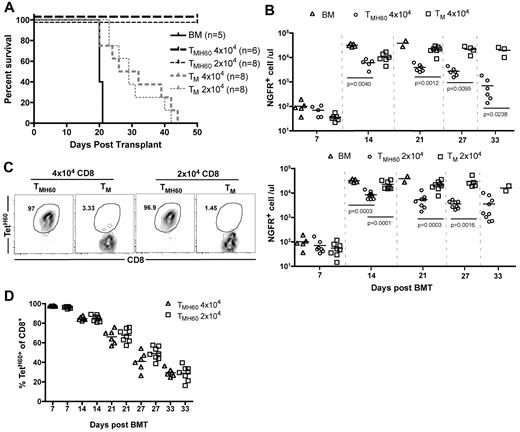
TMH60 were also potent mediators of GVL against mCP-CML. B6.H60 mice were irradiated and reconstituted with C3H.SW BM, B6.H60 mCP-CML with no T cells, or with 4 × 104 or 2 × 104 CD8+ TM from DEC-H60–vaccinated (13% TetH60+) or unmanipulated C3H.SW mice. CD8+ TM from unmanipulated donors prolonged survival as we previously reported,16 but all mice eventually died from mCP-CML (Figure 3A). In contrast, TMH60, containing as few as 2600 TetH60+ cells, were far more potent mediators of GVL as measured by survival (Figure 3A) and numbers of NGFR+ cells in peripheral blood (Figure 3B). TetH60+ cells were again only observed in TMH60 recipients (Figure 3C-D).
TMH60 mediate GVL against mCP-CML. B6.H60 mice were irradiated and reconstituted with C3H.SW BM, B6.H60 mCP-CML cells with no T cells or with 2 × 104 or 4 × 104 CD8+CD44+ TM from DEC-H60–vaccinated or unmanipulated C3H.SW mice. (A) Survival. All deaths were from leukemia. P < .0003 comparing either TMH60 recipient group with its TM control; P ≤ .0002 comparing any TM recipient group with the BM alone control. (B) Numbers of NGFR+ cells in peripheral blood. (C) Representative flow cytometry of TetH60+ cells in peripheral blood at day 7, gated on CD8+ cells. (D) Number of TetH60+ cells in blood of TMH60 recipients at days +7, +14, +21, +27, and +33.
TMH60 mediate GVL against mCP-CML. B6.H60 mice were irradiated and reconstituted with C3H.SW BM, B6.H60 mCP-CML cells with no T cells or with 2 × 104 or 4 × 104 CD8+CD44+ TM from DEC-H60–vaccinated or unmanipulated C3H.SW mice. (A) Survival. All deaths were from leukemia. P < .0003 comparing either TMH60 recipient group with its TM control; P ≤ .0002 comparing any TM recipient group with the BM alone control. (B) Numbers of NGFR+ cells in peripheral blood. (C) Representative flow cytometry of TetH60+ cells in peripheral blood at day 7, gated on CD8+ cells. (D) Number of TetH60+ cells in blood of TMH60 recipients at days +7, +14, +21, +27, and +33.
GVL mediated by TMH60 is antigen specific
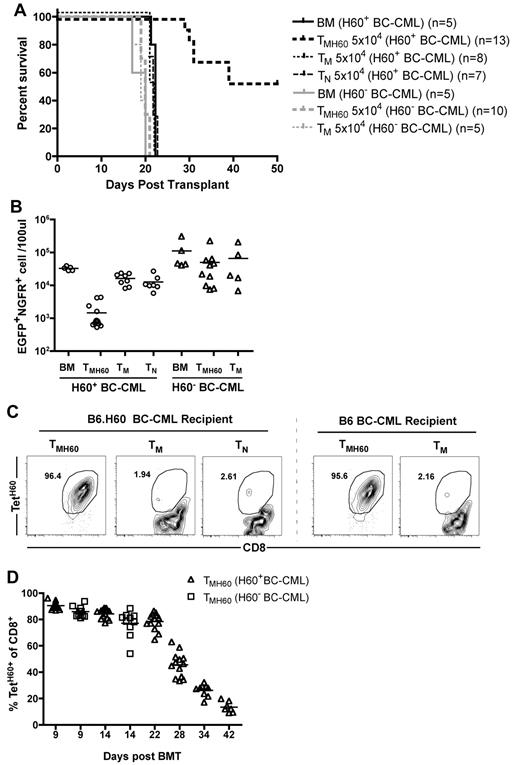
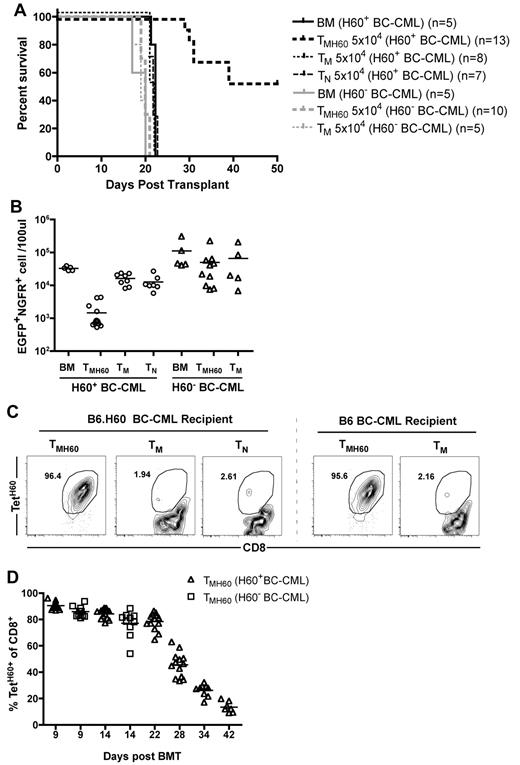
It was possible that GVL was not directed at LTFNYRNL-Kb, but rather at other miHAs to which these T cells may cross-react, as LTFNYRNL-reactive CD8 cells have a relatively diverse TCR repertoire.33 The robust expansion of TMH60 progeny may also have promoted the activation of bystander T cells reactive against other miHAs. To determine whether the TMH60-mediated GVL was H60-specific, we compared GVL against H60+ and H60− mBC-CML. B6.H60 mice were irradiated and reconstituted with C3H.SW BM and wild-type B6 H60− or H60+ mBC-CML cells. Groups of these mice received no T cells or 5 × 104 CD8+ TM from unmanipulated or H60-vaccinated C3H.SW donors. An additional group of H60+ mBC-CML recipients received 5 × 104 CD8+ TN. Only TMH60 prolonged survival relative to BM alone controls, and only against H60+ mBC-CML (Figure 4A). Paralleling the survival data, TMH60 reduced the number of H60+ but not the number of H60− mBC-CML cells (Figure 4B). We observed a similar expansion of LTFNYRNL-reactive CD8 cells in B6.H60 hosts whether the leukemia was H60+ or H60− (Figure 4C-D), indicating that the failure of TMH60 to mediate GVL against H60− mBC-CML was not because of a difference in expansion of H60-reactive CD8 cells. Therefore, the efficacy of TMH60 was because of the direct killing of LTFNYRNL-bearing leukemic targets.
TMH60-mediated GVL requires that the target leukemia expresses H60. B6.H60 mice were irradiated and reconstituted with T cell–depleted C3H.SW BM, B6.H60 or B6 (H60−) mBC-CML cells, with no T cells, or with CD8+ TM from DEC-H60–vaccinated or unmanipulated C3H.SW donors or CD8+ TN from unmanipulated C3H.SW mice. (A) Survival. All deaths were from leukemia. P < .0001 comparing TMH60 recipients of B6.H60 with recipients of B6 mBC-CML cells. (B) Numbers of NGFR+EGFP+ cells in peripheral blood at day 14. P < .0001 comparing TMH60 recipients of B6.H60 versus B6 H60− mBC-CML cells. P = .5 comparing B6 mBC-CML recipients of TMH60 or TM. Recipients of TMH60 mounted similar anti-H60 responses whether they received B6.H60 or B6 mBC-CML cells. (C) Representative flow cytometry. (D) Percentage of peripheral blood CD8+ cells that are TetH60+ in TMH60 recipients at multiple time points.
TMH60-mediated GVL requires that the target leukemia expresses H60. B6.H60 mice were irradiated and reconstituted with T cell–depleted C3H.SW BM, B6.H60 or B6 (H60−) mBC-CML cells, with no T cells, or with CD8+ TM from DEC-H60–vaccinated or unmanipulated C3H.SW donors or CD8+ TN from unmanipulated C3H.SW mice. (A) Survival. All deaths were from leukemia. P < .0001 comparing TMH60 recipients of B6.H60 with recipients of B6 mBC-CML cells. (B) Numbers of NGFR+EGFP+ cells in peripheral blood at day 14. P < .0001 comparing TMH60 recipients of B6.H60 versus B6 H60− mBC-CML cells. P = .5 comparing B6 mBC-CML recipients of TMH60 or TM. Recipients of TMH60 mounted similar anti-H60 responses whether they received B6.H60 or B6 mBC-CML cells. (C) Representative flow cytometry. (D) Percentage of peripheral blood CD8+ cells that are TetH60+ in TMH60 recipients at multiple time points.
Role of miHA tissue distribution
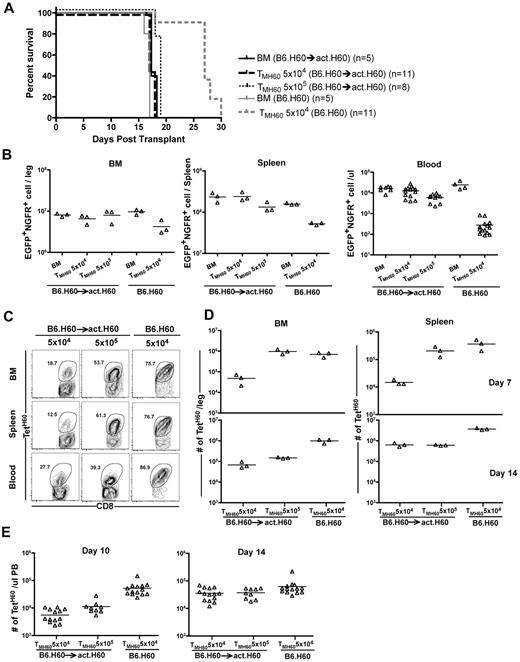
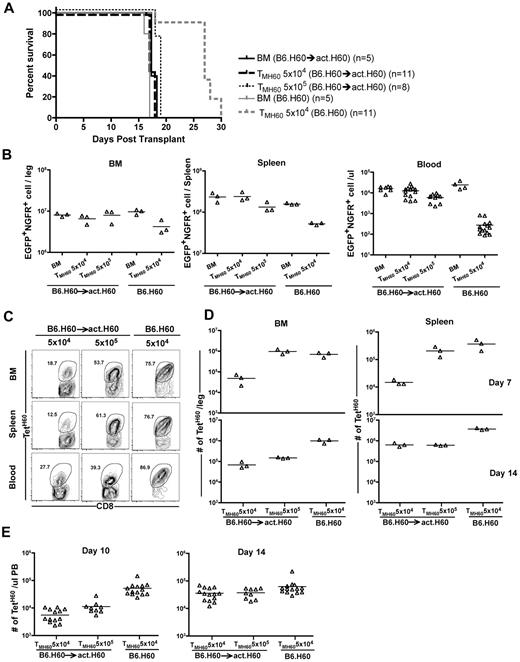
Most miHAs discovered to date are expressed by hematopoietic cells, and this may be at least in part because host hematopoietic cells have been used to propagate T-cell lines used as probes for identifying miHAs.34 However, some of these miHAs have expression relatively limited to hematopoietic cells, whereas others have more widespread expression. To determine whether GVL was affected by differences in expression patterns, we compared GVL mediated by TMH60 in hosts with hematopoietic or ubiquitous expression of H60. We used as hosts in GVL experiments B6.H60 → actH60 (ubiquitous expression) and control B6.H60 hosts (mostly hematopoietic expression25 ). These hosts were irradiated and reconstituted with C3H.SW BM, B6.H60 mBC-CML with no T cells, or 5 × 104 TMH60 (21% TetH60+). An additional group of B6.H60 → actH60 chimeras received 5 × 105 TMH60. BM alone controls died by day 18, whereas survival in B6.H60 recipients of TMH60 was prolonged (Figure 5A). In contrast, TMH60 did not prolong survival in B6.H60 → actH60 hosts, even with 10-fold more TMH60. This survival difference was paralleled by the numbers of mBC-CML cells in peripheral blood, BM, and spleens of mice killed on day 14 and in peripheral blood of mice that were not killed (Figure 5B).
Expression of H60 on host nonhematopoietic cells diminishes TMH60-mediated GVL. B6.H60 or B6.H60 → actH60 chimeras were irradiated and reconstituted with T cell–depleted C3H.SW BM, B6.H60 mBC-CML with no T cells or with 5 × 104 or 5 × 105 CD8+ TM from DEC-H60–vaccinated C3H.SW donors. Cohorts of mice were killed on day +14 for analysis of EGFP+NGFR+ mBC-CML cells and TetH60+ CD8 cells. (A) Survival. All deaths were from leukemia. P < .0001 comparing B6.H60 and B6.H60 → actH60 TMH60 recipients (both TMH60 doses). The survival difference was reflected in the number of EGFP+NGFR+ cells at day +14. P = .1, P = .05, and P < .0001 comparing the numbers of mBC-CML cells in B6.H60 and B6.H60 → actH60 TMH60 recipients in BM, spleen, and peripheral blood, respectively. Peripheral blood data include counts from mice not killed. Fewer TetH60+ CD8 cells were generated in B6.H60 → actH60 chimeras. (C) Representative flow cytometry. (D) Quantitation at days +7 and +14 in BM and spleen. P = .05 comparing B6.H60 recipients of 5 × 104 TMH60 with B6.H60 → actH60 recipients of either 5 × 104 or 5 × 105 TM H60 in spleen and BM at day +14. P = .05 comparing B6.H60 and B6.H60 → actH60 recipients of 5 × 104 TMH60 in spleen and BM at day +7. (E) Quantitation of TetH60+ cells in peripheral blood. P ≤ .02 comparing B6.H60 recipients of 5 × 104 TMH60 with B6.H60 → actH60 recipients of either 5 × 104 or 5 × 105 TMH60. Data are representative of 2 experiments with similar results.
Expression of H60 on host nonhematopoietic cells diminishes TMH60-mediated GVL. B6.H60 or B6.H60 → actH60 chimeras were irradiated and reconstituted with T cell–depleted C3H.SW BM, B6.H60 mBC-CML with no T cells or with 5 × 104 or 5 × 105 CD8+ TM from DEC-H60–vaccinated C3H.SW donors. Cohorts of mice were killed on day +14 for analysis of EGFP+NGFR+ mBC-CML cells and TetH60+ CD8 cells. (A) Survival. All deaths were from leukemia. P < .0001 comparing B6.H60 and B6.H60 → actH60 TMH60 recipients (both TMH60 doses). The survival difference was reflected in the number of EGFP+NGFR+ cells at day +14. P = .1, P = .05, and P < .0001 comparing the numbers of mBC-CML cells in B6.H60 and B6.H60 → actH60 TMH60 recipients in BM, spleen, and peripheral blood, respectively. Peripheral blood data include counts from mice not killed. Fewer TetH60+ CD8 cells were generated in B6.H60 → actH60 chimeras. (C) Representative flow cytometry. (D) Quantitation at days +7 and +14 in BM and spleen. P = .05 comparing B6.H60 recipients of 5 × 104 TMH60 with B6.H60 → actH60 recipients of either 5 × 104 or 5 × 105 TM H60 in spleen and BM at day +14. P = .05 comparing B6.H60 and B6.H60 → actH60 recipients of 5 × 104 TMH60 in spleen and BM at day +7. (E) Quantitation of TetH60+ cells in peripheral blood. P ≤ .02 comparing B6.H60 recipients of 5 × 104 TMH60 with B6.H60 → actH60 recipients of either 5 × 104 or 5 × 105 TMH60. Data are representative of 2 experiments with similar results.
We killed cohorts (not included in the survival curves in Figure 5A) on days 7 and 14 and quantitated TetH60+ cells in spleen and BM, key sites for GVL responses. TetH60+ cells in peripheral blood were enumerated in all mice on days 10 and 14 (Figure 5C-D). Expansion of TetH60+ cells was dramatically blunted in B6.H60 → actH60 mice at all sites and times as compared with B6.H60 recipients, likely accounting for the reduced GVL. The TetH60 response was also blunted in similarly transplanted B6.H60 × actH60 F1 mice (not shown).
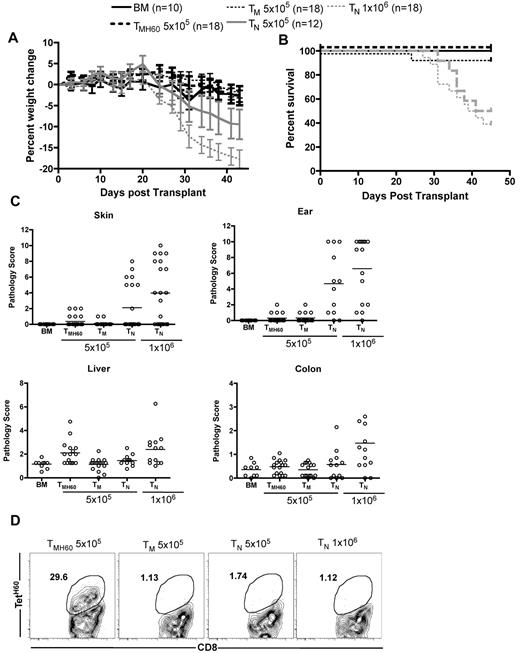
Donor H60 vaccination only increases liver GVHD
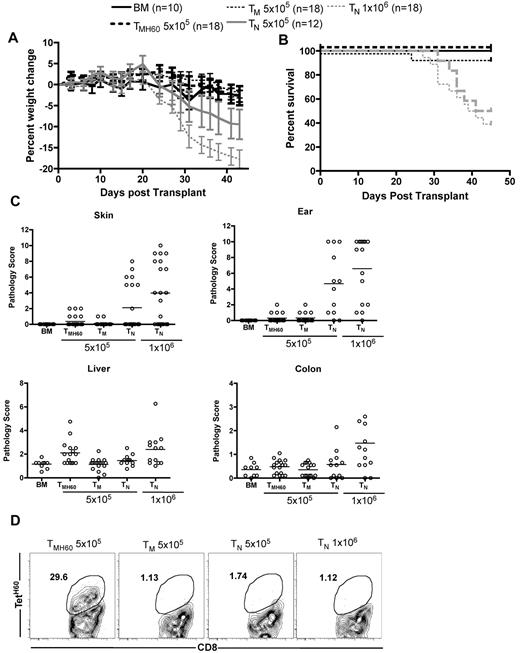
Based on data in Figure 5, targeting a hematopoietically restricted miHA will result in more potent GVL as compared with a ubiquitously expressed antigen. However, methodologies to assess miHA distribution can be unreliable, especially as miHA expression may vary under different conditions. Therefore, it is impossible to ensure that a given targeted miHA is not expressed on nonhematopoietic cells. Therefore, it was clinically important to determine whether donor miHA vaccination would augment GVHD if the targeted miHA was widespread. We therefore compared the ability of C3H.SW TM and TMH60 to induce GVHD in retransplanted B6.H60 → actH60 BM chimeras. B6.H60 → actH60 chimeras were re-irradiated and reconstituted with C3H.SW BM and 5 × 105 CD8+ TM from H60-immune (containing 11% TetH60+ cells) or unmanipulated C3H.SW donors. As positive controls for GVHD, additional groups were transplanted with 5 × 105 or 106 CD8+ TN from unmanipulated C3H.SW donors. As anticipated, CD8+ TN caused weight loss, death, and histopathologic GVHD in H60+ recipients that was similar to that in a small number of control B6 H60− recipients (not shown) and to what we have previously reported in this strain pairing16,23 (Figure 6). In contrast, CD8+ TM from either unmanipulated or H60-vaccinated donors did not cause clinical GVHD as measured by weight loss or death (Figure 6A-B). Neither TM nor TMH60 caused histologic GVHD of the skin, ear, or colon. The only apparent difference between TM and TMH60 was that the latter induced mild histopathologic GVHD of the liver. (Figure 6C), Despite inducing only minimal GVHD, TetH60-reactive T cells expanded in transplanted B6.H60 → actH60 recipients, whereas TetH60+ cells were not found in TM or TN recipients (Figure 6D).
Effect of H60 vaccination on TM recipients. B6.H60 → actH60 mice were irradiated and reconstituted with T cell–depleted C3H.SW BM with no T cells or 5 × 105 CD8+ TM from DEC-H60–vaccinated or unmanipulated C3H.SW donors. Additional positive GVHD control groups received 5 × 105 or 106 TN from unmanipulated C3H.SW donors. As measured by weight change (A) and survival (B), neither CD8+ TM from DEC-H60–vaccinated nor those from unmanipulated C3H.SW mice induced clinical GVHD. P > .07 comparing percent weight change of TMH60 and BM controls at all days except days +3 and +24. P ≥ .29 comparing weight change in TMH60 and TM recipients at all days except day +3. P ≥ .4561 comparing survival in BM alone with the TMH60 or TM group; P ≤ .0109 comparing BM alone recipients with either TN group. Neither TM nor TMH60 cells induced histopathologic skin, ear, or colon GVHD (P > .12 compared with BM alone controls). However, TMH60 induced GVHD in the liver compared with TM or BM alone groups (P = .0015). TM and TMH60 recipients had similar scores in all other tissues (P ≥ .2). In contrast, both TN doses induced significant ear GVHD and 106 TN induced skin, colon, and liver pathology (P ≤ .04 compared with BM alone controls). TetH60+ cells expanded in TMH60 recipients. (D) Representative flow cytometry at day +14. Data are combined from 2 experiments with similar results.
Effect of H60 vaccination on TM recipients. B6.H60 → actH60 mice were irradiated and reconstituted with T cell–depleted C3H.SW BM with no T cells or 5 × 105 CD8+ TM from DEC-H60–vaccinated or unmanipulated C3H.SW donors. Additional positive GVHD control groups received 5 × 105 or 106 TN from unmanipulated C3H.SW donors. As measured by weight change (A) and survival (B), neither CD8+ TM from DEC-H60–vaccinated nor those from unmanipulated C3H.SW mice induced clinical GVHD. P > .07 comparing percent weight change of TMH60 and BM controls at all days except days +3 and +24. P ≥ .29 comparing weight change in TMH60 and TM recipients at all days except day +3. P ≥ .4561 comparing survival in BM alone with the TMH60 or TM group; P ≤ .0109 comparing BM alone recipients with either TN group. Neither TM nor TMH60 cells induced histopathologic skin, ear, or colon GVHD (P > .12 compared with BM alone controls). However, TMH60 induced GVHD in the liver compared with TM or BM alone groups (P = .0015). TM and TMH60 recipients had similar scores in all other tissues (P ≥ .2). In contrast, both TN doses induced significant ear GVHD and 106 TN induced skin, colon, and liver pathology (P ≤ .04 compared with BM alone controls). TetH60+ cells expanded in TMH60 recipients. (D) Representative flow cytometry at day +14. Data are combined from 2 experiments with similar results.
TM from LCMV-immune and LTFNYRNL-vaccinated donors mediate GVL and protect from LCMV infection
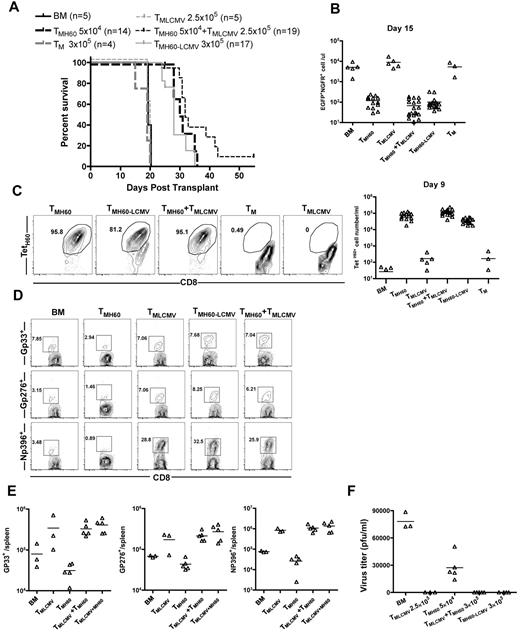
Ideally, TM from miHA-vaccinated donors would both promote GVL and protect from pathogen infection, and we tested this in the H60 system. The CD8+ T-cell response and role of memory CD8 cells in mediating protection from LCMV infection has been intensively studied, and MHCI-tetramer reagents for several dominant epitopes validated, making LCMV ideal for our studies.35 C3H.SW mice were infected with 2 × 105 PFU of LCMV Armstrong. Wild-type mice clear this dose of LCMV and in so doing generate anti-LCMV CD8+ TM. Thirty days later, some LCMV-immune mice were vaccinated with DEC-H60, as were control unmanipulated C3H.SW mice. Seventy days later, these mice were used as CD8+ TM donors in GVL experiments (for experimental design, see supplemental Figure 3). B6.H60 mice were irradiated and reconstituted with C3H.SW BM (from unmanipulated donors) and H60+ mBC-CML cells. Groups of these mice received no T cells or sort-purified CD8+ TM from the following C3H.SW donors: unmanipulated, DEC-H60-vaccinated, LCMV-immune, LCMV-immune and DEC-H60-vaccinated, and a mixture of cells from LCMV-immune and DEC-H60-vaccinated mice.
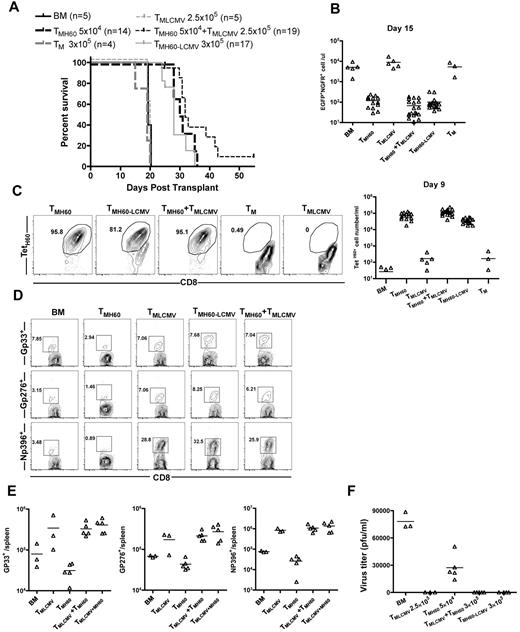
Neither TM from unmanipulated mice nor LCMV-immune donors mediated GVL, as all mice died with the same kinetics as BM alone controls (Figure 7A). In contrast, TM from H60-vaccinated mice, whether they were LCMV-immune mice or were combined with LCMV-immune TM, prolonged survival and reduced the number of mBC-CML cells in peripheral blood (Figure 7A-B). TetH60+ cells were similarly expanded in all recipients of TM from H60-vaccinated donors whether they were derived from mice that were also LCMV-immune or were combined with LCMV-immune cells (Figure 7C). Neither spontaneous TM nor TM from LCMV-immune mice mounted detectable anti-LTFNYRNL responses (Figure 7C). Eighteen days after transplantation, mice were infected with the more virulent LCMV clone 13.36 An additional control group that was transplanted with TM from LCMV-immune mice but without mBC-CML was also challenged. Seven days later, cohorts were killed to quantitate the T-cell response against LCMV and to enumerate viral titers. Recipients of LCMV-immune TM mounted robust LCMV-specific CD8 responses against GP33, GP276, and NP396, which were comparable regardless of whether donors were additionally vaccinated with DEC-H60 or if TM from LCMV-immune and DEC-H60–vaccinated mice were mixed (Figure 7D-E). All recipients of LCMV-immune TM had no detectable serum LCMV, whereas in other mice there were at least 3 × 104 PFU/mL (Figure 7F).
TM from LCMV-immune and H60-vaccinated donors mediate GVL and provide protection from LCMV infection. See supplemental Figure 3 for experimental design. B6.H60 mice were irradiated and reconstituted with C3H.SW BM, B6.H60 mBC-CML cells, with no T cells, or with: 5 × 104 CD8+ TM from DEC-H60-vaccinated or unmanipulated C3H.SW mice; 2.5 × 105 CD8+ TM from LCMV-immune C3H.SW mice; 3 × 105 CD8+ TM from LCMV-immune and DEC-H60-vaccinated mice; or a combination of 5 × 104 CD8+ TMH60 and 2.5 × 105 CD8+ TM from LCMV-immune donors. An additional group of mice was transplanted with 2.5 × 105 TM from LCMV-immune mice without mBC-CML cells. All recipients of TM from LCMV immune donors received ∼ 6 × 103 TetH60+ cells. Cohorts of surviving mice were challenged with LCMV clone 13 on day 18 after transplantation. Cohorts were killed 7 days later to assess the anti-LCMV T-cell response. (A) Survival. P < .0001 comparing TMH60, TMH60-LCMV or TMH60 + TMLCMV recipients with BM alone controls, TM recipients, or TMLCMV recipients. P > .09 comparing TMH60 recipients with TMH60-LCMV or TMH60 + TMLCMV groups. (B) Numbers of NGFR+EGFP+ cells in peripheral blood at day +15. P ≤ .0007 comparing TMH60, TMH60-LCMV or TMH60 + TMLCMV recipients with BM alone controls; P > .07 comparing TM recipients or TMLCMV-cell recipients with BM alone controls. P = .3510 comparing TMH60 recipients with TMH60+LCMV recipients. (C) Expansion of TetH60+ cells in peripheral blood at day +9. Representative flow cytometry (gated on CD8+ cells; left panel) and total numbers of TetH60+ cells (right panel). (D) Representative flow cytometry of NP396, GP276, and GP33 tetramer+ cells in spleen at day +7 after LCMV challenge (gated on CD8 cells). (E) Total numbers of tetramer+ cells. P > .39 comparing TMLCMV recipients with TMH60 + LCMV or TMH60 + TMLCMV recipients for all epitopes. (F) Serum LCMV titers at day 7 after challenge. Data are from 1 of 2 experiments with similar results.
TM from LCMV-immune and H60-vaccinated donors mediate GVL and provide protection from LCMV infection. See supplemental Figure 3 for experimental design. B6.H60 mice were irradiated and reconstituted with C3H.SW BM, B6.H60 mBC-CML cells, with no T cells, or with: 5 × 104 CD8+ TM from DEC-H60-vaccinated or unmanipulated C3H.SW mice; 2.5 × 105 CD8+ TM from LCMV-immune C3H.SW mice; 3 × 105 CD8+ TM from LCMV-immune and DEC-H60-vaccinated mice; or a combination of 5 × 104 CD8+ TMH60 and 2.5 × 105 CD8+ TM from LCMV-immune donors. An additional group of mice was transplanted with 2.5 × 105 TM from LCMV-immune mice without mBC-CML cells. All recipients of TM from LCMV immune donors received ∼ 6 × 103 TetH60+ cells. Cohorts of surviving mice were challenged with LCMV clone 13 on day 18 after transplantation. Cohorts were killed 7 days later to assess the anti-LCMV T-cell response. (A) Survival. P < .0001 comparing TMH60, TMH60-LCMV or TMH60 + TMLCMV recipients with BM alone controls, TM recipients, or TMLCMV recipients. P > .09 comparing TMH60 recipients with TMH60-LCMV or TMH60 + TMLCMV groups. (B) Numbers of NGFR+EGFP+ cells in peripheral blood at day +15. P ≤ .0007 comparing TMH60, TMH60-LCMV or TMH60 + TMLCMV recipients with BM alone controls; P > .07 comparing TM recipients or TMLCMV-cell recipients with BM alone controls. P = .3510 comparing TMH60 recipients with TMH60+LCMV recipients. (C) Expansion of TetH60+ cells in peripheral blood at day +9. Representative flow cytometry (gated on CD8+ cells; left panel) and total numbers of TetH60+ cells (right panel). (D) Representative flow cytometry of NP396, GP276, and GP33 tetramer+ cells in spleen at day +7 after LCMV challenge (gated on CD8 cells). (E) Total numbers of tetramer+ cells. P > .39 comparing TMLCMV recipients with TMH60 + LCMV or TMH60 + TMLCMV recipients for all epitopes. (F) Serum LCMV titers at day 7 after challenge. Data are from 1 of 2 experiments with similar results.
Discussion
Using clinically relevant leukemia models, we demonstrate a straightforward method for augmenting GVL that overcomes the technical complications and hazards inherent to adoptive cell therapy with T-cell lines or antigen receptor–modified T cells. In contrast to the limited expansion and survival of ex vivo–expanded T cells, H60-reactive TM underwent dramatic in vivo expansion on transfer. We did not observe any immediate toxicity after TMH60 injection, even when hosts ubiquitously expressed H60, as has been reported when large numbers of miHA-reactive CD8 cells were infused in humans.6 This could be because of the relatively small number of H60-reactive cells transferred and to their being memory cells, as memory cells must be activated by professional APCs37 and without such activation are unlikely to have immediate effector function. Because we infused all CD8+ TM, protective antipathogen immunity was also transferred without further ex vivo cell engineering.
The genetically modified LTFNYRNL-encoding anti–DEC-205 Ab was a novel and efficient tool for vaccination that generated miHA-reactive TM with a mostly central memory phenotype. Consistent with their central memory phenotype, upon transfer, LTFNYRNL-reactive cells among TMH60 underwent dramatic expansion and effector differentiation manifest by IFN-γ production and the direct killing of H60+ but not H60− mBC-CML cells. The ability to undergo such clonal expansion allowed a remarkably small number of LTFNYRNL-specific TM to efficiently mediate GVL against 2 realistic leukemia models. As few as 2.6 × 103 TetH60+ TM completely protected mice from mCP-CML, whereas more than 106 unfractionated or naive CD8 cells from unmanipulated donors were required for a similar effect. Likewise, the transfer of approximately 5 × 103 TetH60+ cells mediated GVL against GVL-resistant mBC-CML. Given the potency of miHA-reactive TM, the donor vaccination approach has promise for application in humans even if the frequency of miHA-reactive TM achieved is not as high as in our studies. Another clinically relevant property of TMH60 was that they expanded and were effective without CD4 help. Therefore, CD8+ TM from miHA-vaccinated donors could be administered without CD4 cells, which may further reduce the potential for GVHD.
TMH60 were readily generated in LCMV-immune mice, which parallels the proposed clinical approach of vaccinating pathogen-immune donors, without compromising the performance of either miHA-reactive or virus-reactive TM. TM from LCMV-immune and DEC-H60–vaccinated mice mediated GVL similarly, as did TM from mice only vaccinated against H60. Likewise, TM from LCMV-immune plus DEC-H60–vaccinated donors protected recipients from LCMV similarly to TM from mice that were only LCMV immune.
There was no evidence for cross-reactivity of LCMV-immune TM against H60 or H60-reactive TM against LCMV (Figure 7C-E) despite LCMV infection and LTFNYRNL engaging T cells with a relatively broad distribution of TCR Vβ usage, including some that overlap.33,38,39 H60-reactive TM also did not cross-react with other miHAs in that TMH60 did not mediate GVL against H60− leukemias and expansion of TetH60+ cells only occurred if the recipient expressed H60, even though C3H.SW donors and B6 recipients differ by numerous miHAs. These data suggest that virus-immune CD8+ TM are less likely to recognize miHAs than TN and that miHA-specific TM generated by vaccination are unlikely to cross-react with other miHAs. These properties could diminish the risk that CD8+ TM from pathogen-immune donors vaccinated against a hematopoietically restricted, MHCI-restricted miHA will cause serious GVHD.
Fontaine et al have also explored MHCI-restricted miHA-vaccination to promote GVL.40 Our studies differ from this work in several notable ways. Most significantly, Fontaine et al studied the transfer of unfractionated spleen cells from mice immunized only 14 days previously. Certainly, the miHA-reactive cells transferred were predominated by newly activated effector T cells, as 14 days is insufficient for TM differentiation.41 This may at least in part explain the rather large numbers of miHA-reactive T cells required for GVL, the relatively small magnitude of expansion after transfer, and why donor vaccination augmented GVHD. In contrast, the H60-reactive T cells in our studies were true memory cells, mostly with a central memory phenotype. We also transferred purified CD8+ TM rather than total spleen cells. This parallels how such an approach would be translated to the clinic and allowed us to isolate the effects of transferred TM, independent of contributions from naive CD8 cells or CD4 cells that were cotransferred in the prior studies.
Effective TM-mediated GVL required that the target antigen be hematopoietically restricted. In a transplantation model with only a single miHA mismatch, a previous study reported that miHA-reactive effector T cells more potently killed leukemia cells when only the leukemia cells expressed the targeted antigen.42 That transferred cells were effectors may explain their ability to kill leukemia cells without endogenous host miHA-expressing APCs, which have been shown to be required for GVL in allogeneic BM transplantation systems.15,43-45 Asakura et al recently reported that GVL was reduced when host miHAs were widespread relative to when they were hematopoietic.46 Our data confirm and extend their findings. They could not specifically track miHA-reactive and GVL-mediating CD8 cells, and therefore it was possible that reduced GVL was because of T-cell dysfunction rather than reduced numbers. Because we could track GVL-inducing T cells, we were able to establish that the reduced GVL was because of fewer LTFNYRNL-reactive CD8 cells when H60 was ubiquitous. That the number of TetH60+ cells was reduced in spleen and lymph nodes (not shown) in B6.H60 → actH60 mice suggests that the key H60-expressing nonhematopoietic cells that suppress the anti-H60 response are located in secondary lymphoid tissues. This remains to be further explored. In a delayed T-cell infusion model, Fluttes et al also found alloreactive T-cell responses to be diminished when antigen was widespread.47
To maximize GVL efficacy and to minimize GVHD,48 it would be ideal if targeted miHAs were restricted to the hematopoietic system. However, it is difficult to accurately assess miHA distribution, especially as a given gene may only be expressed in a subset of cells or may be induced only under specific conditions, perhaps even by the alloimmune response itself. Although the outcome may vary depending on the specific miHA targeted, it is reassuring that GVHD in mice with ubiquitous H60 expression was only minimally augmented when CD8+ TM came from H60-vaccinated donors, even though we infused ∼ 10-fold more TetH60+ cells than in our GVL experiments. The failure of TMH60 to mediate more severe GVHD in B6.H60 → actH60 hosts was not because of a failure of H60-reactive cells to expand, although expansion of TetH60+ cells was blunted as compared with that in transplanted B6.H60 mice.
In summary, these studies establish a promising approach for augmenting GVL that does not require elaborate cell-processing facilities that are unavailable at most centers. However, the success of donor vaccination will depend on the availability of a sufficient number of miHAs with allele frequencies such that an appropriate miHA is available for a meaningful fraction of donor/recipient pairs. New biocomputational approaches for identifying miHAs have accelerated the pace of miHA identification,34,49,50 making it reasonable to propose that appropriate candidate miHAs will be identified for many patients in the near future.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Yale University Animal Resources staff for excellent animal care.
This work was supported by a grant from the Burroughs Wellcome Fund and by the National Institutes of Health (grant CA096943). W.D.S. was supported by a Clinical Scholar Award from the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: N.L. designed and performed the experiments, analyzed the data, and wrote the manuscript; H.Z. and C.M.-M. developed critical techniques and performed the experiments; S.V. created the DEC-H60 construct; W.C. and S.K. assisted in the experiments with LCMV infection; H.S.T. performed the experiments; D.R. created the H60 transgenic and congenic mice and provided critical advice on experimental design; A.J.D. and J.M. scored the GVHD histopathology; and W.D.S. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Warren D. Shlomchik, Yale University School of Medicine, 333 Cedar St, PO Box 208032, New Haven CT, 06520-8032; e-mail: warren.shlomchik@yale.edu.