In this issue of Blood, Ehrhardt and colleagues clearly demonstrate antagonistic effects when anthracyclins and vinca alkloids are used simultaneously.1 These findings challenge us to take a fresh look at how drugs are combined when treating patients with hematologic malignancies.
Combination chemotherapy has formed the basis for the treatment of a range of hematologic malignancies since the 1960s and there is no doubt that it has resulted in a dramatic improvement in response rates and survival. Despite this success, significant numbers of tumors are resistant to or recur after treatment. In recent years research attention has turned away from traditional chemotherapeutic agents in the search of novel strategies and compounds. While this is appropriate, Ehrhardt et al have demonstrated that we may be able to get considerably more from traditional agents using better-informed schedules, and that our current regimes may even, at times, be counterproductive.
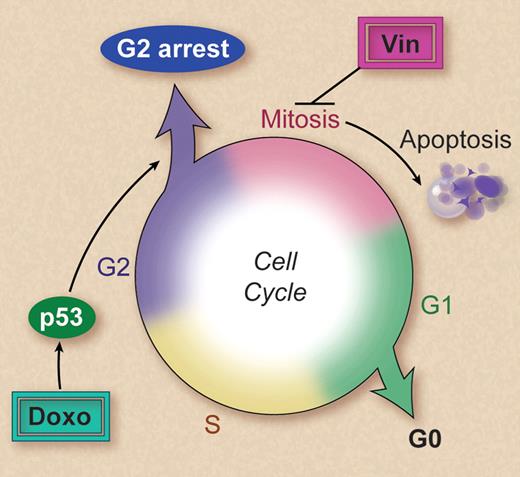
Vinca alkaoids and anthracyclines are two of the most commonly used chemotherapeutic agents for hematologic malignancies, usually being administered on the same day (see http://clinicaltrials.gov for examples). Alarmingly, this study by Ehrhardt and colleagues demonstrates that anthracyclines inhibit apoptosis induced by vinca alkaloids. This finding applies to all drugs tested from each class and is highly dependent on the timing of exposure to each agent, with the pre-exposure to anthracyclines having the greatest antagonistic effect. The investigators found that this was not the result of direct drug-drug interactions but persisted after the anthracycline had been removed. Most significantly, administration of doxorubicin the day before vincristine reduced the therapeutic effect of vincristine in a xenograft model of human T-cell acute lymphoblastic leukemia, providing strong evidence that the effect is likely to be occurring in patients. It is perhaps not surprising that anthracycline-induced p53-dependent cell-cycle arrest in the G2 phase of the cell cycle2,3 was involved in preventing vinca alkaloid–induced cell death (see figure). After all, vinca alkaloid–induced cell death is associated with microtubule disruption during mitosis.4 Why hasn't this potential interaction been considered before? It is always easier to see the obvious in hindsight, and the mechanisms of action of the majority of chemotherapeutic agents are far from fully understood. The mechanistic insights provided by Ehrhardt et al's study immediately raise questions about whether other drugs that activate p53 will have similar antiapoptotic activities when administered with or before additional cell cycle–dependent drugs. Because most chemotherapeutic agents both activate p53 and are cell cycle–dependent, it is likely that similar negative interactions will be identified.
Vincristine (Vin) triggers apoptosis subsequent to disruption of the mitotic spindle during mitosis. Doxorubicin (doxo) activates p53, resulting in a cell cycle arrest in the G2 phase. This prevents cells entering mitosis, thereby avoiding the cytotoxic effects of vincristine. Professional illustration by Debra T. Dartez.
Vincristine (Vin) triggers apoptosis subsequent to disruption of the mitotic spindle during mitosis. Doxorubicin (doxo) activates p53, resulting in a cell cycle arrest in the G2 phase. This prevents cells entering mitosis, thereby avoiding the cytotoxic effects of vincristine. Professional illustration by Debra T. Dartez.
The way that chemotherapeutic agents are combined in the clinic is the result of decades of clinical trials aimed at refining dosing schedules. However, this approach is limited by the number of trials that can be conducted, and today, with newer agents taking center stage, few trials tweaking the timing of established drugs are likely to be conducted. Ehrhardt and colleagues demonstrate that careful evaluation of the effects of drug combinations can be assessed in a methodical manner using relatively simple in vitro studies, and confirmed where appropriate in preclinical models. While this study examined anthracyclines and vinca alkaloids, how much do we really know about the interactive effects that occur in combination chemotherapy? While synergistic interactions are regularly published, a literature search uncovered only one or two references, beyond the handful cited by Ehrhardt and colleagues, where negative interactions have been reported. Together these reports cover schedule-dependent antagonism between methotrexate and cytarabine5 and between methotrexate and L-asparaginase, as well as a mixture of synergistic, additive, and antagonistic interactions between prednisolone and vincristine, mafosfamide, or daunorubicin.6 Potentially antagonistic combinations of these agents are still commonly applied in the clinic.
Another layer of complexity to consider is inter-patient and/or disease-specific variations in responses. In this study by Ehrhardt et al not all patient samples responded in the same way, a finding previously noted by others,6 with a small group of patient samples demonstrating synergistic killing when exposed to exactly the same drug schedule that produced the reported antagonism. So how can we predict the response of individual patients? There was no observed association between patient cytogenetic abnormalities and response. The involvement of p53 in anthracycline-induced vinca alkaloid resistance would suggest that mutation or loss of p53, which is uncommon in hematologic malignancies6 could be responsible; however, p53 loss or mutation was not enriched in this group of patients. These patients could have other defects in the DNA damage response such as mutations in Ataxia telangiectasia, CHK2 checkpoint homolog (CHEK2), or meiotic recombination 11 homolog A (MRE11) genes,7,8 preventing cell-cycle arrest and therefore exposing cells to the cytotoxic actions of vincristine. However, much needs to be learned before we can tailor chemotherapy schedules to obtain maximal responses for all patients.
Why is there a paucity of studies demonstrating negative interactions? This is likely to be at least partly because of publication bias against what superficially appears to be negative data. Productivity and funding are vital to almost all research laboratories, ensuring the pursuit of the most promising work and a resultant neglect of the negative, difficult-to-explain, or inconvenient data. It may be particularly important to consider negative interactions as newer agents, such as kinase inhibitors, are incorporated into current schedules, as many of these agents have major cytostatic functions. While this study by Ehrhardt and colleagues elegantly defines the antagonistic mechanism between anthracyclines and vinca alkaloids, it remains to seen whether similar studies investigating other key chemotherapeutic combinations are conducted and published, and more importantly how these findings are translated into clinical practice.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■