Abstract
Application of anthracyclines and Vinca alkaloids on the same day represents a hallmark of polychemotherapy protocols for hematopoietic malignancies. Here we show, for the first time, that both drugs might act most efficiently if they are applied on different days. Proof-of-concept studies in 18 cell lines revealed that anthracyclines inhibited cell death by Vinca alkaloids in 83% of cell lines. Importantly, in a preclinical mouse model, doxorubicin reduced the anti–tumor effect of vincristine. Both drugs acted in a sequence-dependent manner and the strongest anti–tumor effect was obtained if both drugs were applied on different days. Most notably for clinical relevance, in 34% of 35 fresh primary childhood leukemia cells tested in vitro, doxorubicin reduced the anti–tumor effect of vincristine. As underlying mechanism, doxorubicin activated p53, p53 induced cell-cycle arrest, and cell-cycle arrest disabled inactivation of antiapoptotic Bcl-2 family members by vincristine; therefore, vincristine was unable to activate downstream apoptosis signaling. As molecular proof, antagonism was rescued by knockdown of p53, whereas knockdown of cyclin A inhibited vincristine-induced apoptosis. Our data suggest evaluating anthracyclines and Vinca alkaloids on different days in future trials. Selecting drug combinations based on mechanistic understanding represents a novel conceptional strategy for potent polychemotherapy protocols.
Introduction
Chemotherapeutic drugs, such as anthracyclines and Vinca alkaloids, are used during anti–tumor therapy with the aim of clearing tumor cells on induction of cell death. As monotherapy is poorly effective, chemotherapeutic drugs are combined in polychemotherapy protocols to increase anti–tumor efficiency.1-3
Combinations of different chemotherapeutic drugs have been optimized heuristically in clinical multicenter trials. Because of substantial efforts required to realize clinical trials, only a limited number of drug combinations and application parameters could be optimized clinically. For example, anthracyclines and Vinca alkaloids are widely coapplied on the same day in many different anti–cancer protocols to treat hematopoietic malignancies and some forms of solid tumors. Nevertheless, the application schedule and sequence of these 2 classes of chemotherapeutic drugs were never optimized in clinical trials.4,5 The rationale for using both drugs in combination was based on very limited (< 10) animal experiments back in the 1970s, which were performed exclusively on rat and mouse tumor cells, as far as we know.6,7
For certain chemotherapeutic drug combinations, anti–tumor efficiency highly depends on the application schedule, as shown in few clinical trials and numerous preclinical studies.8-13 Among others, sequence dependency was proven for the combination of asparaginase and methotrexate for anti–leukemia therapy.8,10,12-16
On a molecular level, the effects and signaling pathways induced by many chemotherapeutic drugs have not been analyzed in depth, and the consequences of their combinatorial application remain unclear.17 As far as we know, no mechanistic data exist so far to explain the sequence dependency of any clinically proven drug combination on a molecular level.
Here we aimed at optimizing the anti–tumor effect of the drug combination of anthracyclines and Vinca alkaloids based on an understanding of their signaling interactions. We detected a strongly sequence-dependent anti–tumor effect of both drugs and characterized the intracellular signaling mechanisms responsible for sequence dependency in leukemia cells. We introduced an optimized, mechanism-based application schedule, which might improve the effectiveness of both drugs in clinical trials for hematopoietic malignancies.
Methods
Materials
For Western blot, the following antibodies were used: anti–Bcl-xL, anti–Casp-2, anti–cleaved Casp-3, anti–cleaved Casp-6, anti–cleaved Casp-7, anti–cleaved PARP, and anti–phospho-histone H3 (Ser10) from Cell Signaling Technology; anti–Bcl-2, anti–cleaved Casp-1, and anti–p53 from Santa Cruz Biotechnology; anti–cyclin A and anti–GAPDH from Thermo Fisher; anti–Casp-9 from Transduction Laboratories; anti–Casp-10 from MBL; and anti–caspase-8 from Alexis Corp. For flow cytometric analysis, annexin V was obtained from BD Biosciences and anti–p-Histon H3 Ser 10 from Cell Signaling Technology. Vincristine (VCR) and all biochemical inhibitors were obtained from Calbiochem with the exception of 2,3-dichlorophenoxypropylaminoethanol (DCPE; Biomol International LP). All other reagents were obtained from Sigma-Aldrich.
Cell lines, transfection experiments, and primary samples
All leukemia cell lines were obtained from DSMZ and were maintained as previously described.18,19 For all cell line experiments, cells were seeded at 0.2 × 106/mL and incubated with chemotherapeutic drugs at peak plasma concentration for 48 hours unless otherwise stated. CFUs were performed with a starting cell density of 0.02 × 106/mL using methylcellulose base media supplemented with 2mM l-glutamine, 10mM HEPES buffer solution, and 50 U/mL penicillin and 50 μg/mL streptomycin (Invitrogen). Transfection experiments were performed using the Cell Line Nucleofector kit V (Lonza Walkersville) according to the manufacturer's instructions and shRNA against p53 and mock plasmids as previously described.19 Alternatively, lentiviral transduction was performed targeting the identical p53 sequence or cyclin A with the following oligonucleotides annealed: sense sequence 5′-GATCCGAAATGTACCCTCCAGAAATTGAATTCGTTTCTGGAGGGTACATTTCTTTTTG-3′, antisense sequence 5′-AATTCAAAAAGAAATGTACCCTCCAGAAACGAATTCAATTTCTGGAGGGTACATTTCG-3′.
Primary leukemia blasts were obtained from 35 children treated for acute leukemia at the Ludwig Maximilian University Children's Hospital and the Children's Hospital of TU Munich during 2005 and 2008. Samples were obtained, isolated, and stimulated simultaneously with doxorubicin and VCR as previously described.19,20
Animal trial
The animal trial was approved by the Bavarian federal government, and animal care was in accordance with institution guidelines. Female NSG mice 8 to 12 weeks of age were obtained from Charles River. Mice were subcutaneously injected with 0.1 mL PBS containing 2.8 × 106 CEM cells into both flanks. After 10 days, all animals had developed tumors with diameters ranging between 2 and 6 mm. The animals were distributed into groups (control, n = 8 animals; doxorubicin, n = 8; VCR, n = 16; doxorubicin + VCR, n = 19) and treated with intravenous injections of doxorubicin solution (0.3 mg/kg body weight; MEDAC) and/or the intravenous administration of VCR solution (0.9 mg/kg body weight; TEVA) at day 0 and 8. Tumor size (2 dimensions) was determined at the beginning of treatment and was followed for 15 days. The relative change in tumor size was calculated for each animal.
Cell imaging, flow cytometric analysis, apoptosis assays, and Western blot analysis
For biochemical inhibition, cell lines were pretreated for 8 hours or irradiated 24 hours before further stimulation for another 48 hours. The release of cytochrome c was detected as recently described,18 with loss of mitochondrial membrane potential using DiOC6 staining. Cell-cycle analysis was performed using propidium iodide staining. To discriminate between G2 and M arrest, double staining for p-histone H3 and propidium iodide was performed. In brief, cells were fixed by 70% ethanol, resuspended in PBS with 0.25% Triton X, followed by incubation with specific antibody in PBS with 1% BSA and addition of RNAse A (100 μg/mL) and propidium iodide 20 μg/mL after 3 washing steps. Apoptosis was measured by forward side scatter analysis and precision of this technique confirmed by annexin V and propidium iodide double staining according to the manufacturer's instructions using FACscan or LSR II flow cytometry and Cell Quest Pro Version 3.2.1 (BD Biosciences) and FlowJo Version 8.3 (TreeStar) software. Western blot analysis of total cellular protein or of cytosolic and nuclear fractions was performed as previously described, and 10 μg of protein was loaded.19
Statistical analysis
Specific apoptosis was calculated as [(apoptosis of stimulated cells at end minus apoptosis of unstimulated cells at end) divided by (100 − apoptosis of unstimulated cells at end) × 100], specific survival as [100 − specific apoptosis induction]. In Figure 2C, doxorubicin resistance was defined as specific apoptosis of < 10%. In Figure 2C, fractional product method (FP)21 was used to discriminate between synergistic and antagonistic apoptosis induction after combined application of doxorubicin and VCR. FP values ≤ −0.1 were defined as relevant antagonism, and FP values ≥ 0.1 as relevant synergism. For primary samples, the expected apoptosis induction of independent application of doxorubicin and VCR was calculated as [(1 − (survival after simulation with doxorubicin × survival after stimulation with VCR)) × 100]. Alternatively, median effect blots were used performed by CompuSyn Version 1.0 software.
For cell line experiments, data are presented as the mean values of at least 3 independent experiments ± SEM unless otherwise stated. To test for significant differences, the paired t test was applied; for multivariate analysis, 1-way rank-sum ANOVA was used. Significance was set at P < .05. For animal trials, Mann-Whitney rank-sum test or Student t test was applied for P < .01.
Results
Anthracyclines and Vinca alkaloids are applied on the same day in several polychemotherapy protocols for hematopoietic malignancies. Here, we aimed at optimizing their anti–tumor effect based on the understanding of the responsible signaling interaction.
Inhibition of Vinca alkaloid-induced apoptosis by anthracyclines
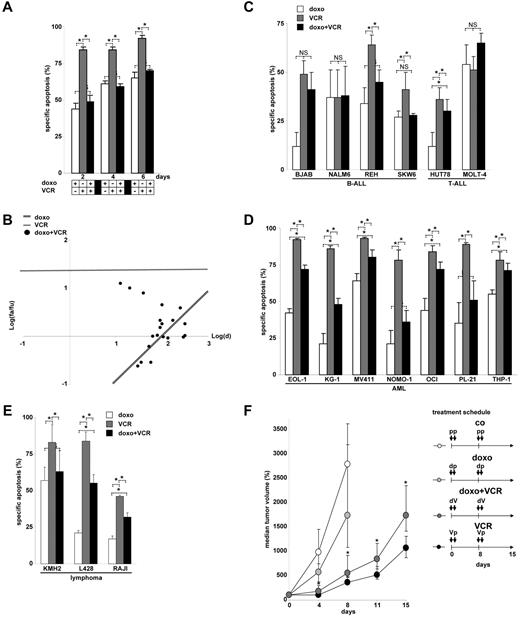
The T-cell leukemia cell lines CEM (data presented in Figures 1,Figure 2,Figure 3,Figure 4,Figure 5,Figure 6–7 and supplemental Figures 1-3, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and JURKAT (data presented in supplemental Figure 4) are highly sensitive toward VCR-induced apoptosis and partially sensitive toward doxorubicin-induced apoptosis. To our surprise and as a completely new finding, doxorubicin inhibited VCR-induced apoptosis in both cell lines, when both drugs were given simultaneously (Figure 1A). The net effect of the combinatorial use of both drugs resulted in antagonism as measured by various techniques, including morphology, forward side scatter analysis in FACscan, annexin V staining, uptake of propidium iodide, and DNA fragmentation (supplemental Figure 1). Both median effect plots and the fractional product method confirmed antagonistic interaction between doxorubicin and VCR (Figure 1B; and data not shown). More detailed studies revealed that doxorubicin inhibited VCR-induced apoptosis over a long period of time, in a dose-dependent manner, and enabled the survival of colony-forming tumor cells otherwise erased by VCR (Table 1; supplemental Table 1). The antiapoptotic effect of doxorubicin was not because of direct drug-drug interaction as it persisted after medium exchange. The antiapoptotic effect was not restricted to doxorubicin but also observed with other anthracyclines, including daunorubicin, epirubicin, and idarubicin. Reciprocally, doxorubicin attenuated induction of cell death not only by VCR, but also by vinblastine and vinorelbine, suggesting a general inhibitory and antiapoptotic effect of anthracyclines toward Vinca alkaloid-induced cell death (data not shown for all).
Inhibition of Vinca alkaloid-induced apoptosis by anthracyclines. (A) CEM leukemia cells were simultaneously stimulated with doxorubicin (doxo, 100 ng/mL) and VCR (300 ng/mL) for time periods indicated. *P < .05 (ANOVA). NS indicates not significant. (B) Corresponding data from panel A for 48-hour incubation time and simultaneous application were analyzed for a total of n = 20 combinations by median effect plots investigating a range of drug concentrations (doxorubicin 10, 30, 60, and 100 ng/mL; VCR 3, 10, 30, 100, and 300 ng/mL). fa indicates apoptotic fraction; fu, fraction of cells alive; and d, drug dosage. (C-E) Further n = 6 B-ALL and T-ALL (C), n = 7 acute myeloid leukemia (D), and n = 3 lymphoma cell lines (E) were stimulated with doxorubicin and VCR for 48 hours as in panel A. *P < .05 (ANOVA). NS indicates not significant. (F) Xenograft study of CEM leukemia cells subcutaneously implanted into NSG mice was performed as described in “Animal trial.” Mice were treated as shown in the treatment schedule with doxorubicin (0.3 mg/kg) and/or VCR (0.9 mg/kg) or placebo as shown. Tumor size was measured in 2 dimensions, and tumor volume was calculated. Statistical analysis using Mann-Whitney rank-sum test was performed comparing VCR and combinatorial treatment (doxorubicin + VCR) at each measurement point (*P < .01) and revealed that doxorubicin followed by VCR 1 day later significantly inhibited the effect of VCR alone. The 25th and 75th quartiles are shown. p indicates placebo; d, doxorubicin; and V, VCR.
Inhibition of Vinca alkaloid-induced apoptosis by anthracyclines. (A) CEM leukemia cells were simultaneously stimulated with doxorubicin (doxo, 100 ng/mL) and VCR (300 ng/mL) for time periods indicated. *P < .05 (ANOVA). NS indicates not significant. (B) Corresponding data from panel A for 48-hour incubation time and simultaneous application were analyzed for a total of n = 20 combinations by median effect plots investigating a range of drug concentrations (doxorubicin 10, 30, 60, and 100 ng/mL; VCR 3, 10, 30, 100, and 300 ng/mL). fa indicates apoptotic fraction; fu, fraction of cells alive; and d, drug dosage. (C-E) Further n = 6 B-ALL and T-ALL (C), n = 7 acute myeloid leukemia (D), and n = 3 lymphoma cell lines (E) were stimulated with doxorubicin and VCR for 48 hours as in panel A. *P < .05 (ANOVA). NS indicates not significant. (F) Xenograft study of CEM leukemia cells subcutaneously implanted into NSG mice was performed as described in “Animal trial.” Mice were treated as shown in the treatment schedule with doxorubicin (0.3 mg/kg) and/or VCR (0.9 mg/kg) or placebo as shown. Tumor size was measured in 2 dimensions, and tumor volume was calculated. Statistical analysis using Mann-Whitney rank-sum test was performed comparing VCR and combinatorial treatment (doxorubicin + VCR) at each measurement point (*P < .01) and revealed that doxorubicin followed by VCR 1 day later significantly inhibited the effect of VCR alone. The 25th and 75th quartiles are shown. p indicates placebo; d, doxorubicin; and V, VCR.
Cell lines of different hematopoietic tumors were tested, including 8 B-acute lymphoblastic leukemia (B-ALL) and T-ALL cell lines, 7 acute myeloid leukemia cell lines, and 3 lymphoma cell lines. All lines expressed functionally active p53.22 Overall, in 15 of 18 (83%) cell lines, a negative interaction between doxorubicin and VCR was detected, when both drugs were applied together, as their mutual apoptosis induction was lower than expected from the activity from the single drugs (Figure 1A,C-E; supplemental Figure 4A). When hematopoietic subtypes were analyzed, inhibition of VCR-induced apoptosis was present in 63% of ALL and 100% of acute myeloid leukemia and lymphoma cell lines.
To test the described phenotype within the complex in vivo situation, NSG mice were xenografted subcutaneously with human CEM T-ALL leukemia cells. CEM cells bearing mice were treated with either doxorubicin or VCR alone or both drugs simultaneously. Similar to the in vitro data, doxorubicin significantly inhibited the anti–tumor effect of VCR in vivo, when doxorubicin was applied together with VCR (Figure 1F).
In summary, doxorubicin frequently and severely inhibited VCR-induced apoptosis in hematopoietic tumor cells in vitro and in vivo when both drugs were applied simultaneously.
Antagonistic effect of doxorubicin and VCR on primary leukemic tumor cells
To approximate the clinical situation and move beyond established cell lines, 35 fresh primary tumor samples were investigated. Cells were obtained from children with acute leukemia and isolated from diagnostic bone marrow aspirations before the onset of anti–cancer treatment. Fresh primary leukemia cells were stimulated in vitro with each drug alone or both drugs simultaneously, applying a range of clinically relevant drug concentrations. Doxorubicin inhibited VCR-induced apoptosis in several primary leukemia tumor cells (Figure 2A) and over a broad range of concentrations (Figure 2B).
Antagonistic effect of doxorubicin and VCR on primary leukemic tumor cells. (A) Three primary leukemia samples (patients 6, 10, and 12 from panel C) were simultaneously stimulated with doxorubicin (300 ng/mL) and VCR (300 ng/mL). Apoptosis induction was measured after 48 hours, when spontaneous apoptosis had reached 40%, otherwise after 72 hours. (B) Patient sample 10 (left panel, VCR 300 ng/mL) and patient 6 (right panel, VCR 30 ng/mL) from panel A were stimulated with doxorubicin and VCR (as indicated). (C) Thirty-five primary leukemia samples were stimulated with either doxorubicin or VCR alone or simultaneously with doxorubicin and VCR as in panel A. All samples were sensitive for doxorubicin (specific apoptosis > 10%) besides samples 29 to 32. Measured apoptosis for the combination of doxorubicin and VCR is depicted as black dots (called “simultaneous application”). The expected apoptosis induction, if doxorubicin and VCR were given independently, was calculated from the results obtained with each drug alone as described in “Statistical analysis” and is shown as white and gray bars (called “independent application”). Definition of antagonistic, additive, and synergistic apoptosis is described in “Statistical analysis.”
Antagonistic effect of doxorubicin and VCR on primary leukemic tumor cells. (A) Three primary leukemia samples (patients 6, 10, and 12 from panel C) were simultaneously stimulated with doxorubicin (300 ng/mL) and VCR (300 ng/mL). Apoptosis induction was measured after 48 hours, when spontaneous apoptosis had reached 40%, otherwise after 72 hours. (B) Patient sample 10 (left panel, VCR 300 ng/mL) and patient 6 (right panel, VCR 30 ng/mL) from panel A were stimulated with doxorubicin and VCR (as indicated). (C) Thirty-five primary leukemia samples were stimulated with either doxorubicin or VCR alone or simultaneously with doxorubicin and VCR as in panel A. All samples were sensitive for doxorubicin (specific apoptosis > 10%) besides samples 29 to 32. Measured apoptosis for the combination of doxorubicin and VCR is depicted as black dots (called “simultaneous application”). The expected apoptosis induction, if doxorubicin and VCR were given independently, was calculated from the results obtained with each drug alone as described in “Statistical analysis” and is shown as white and gray bars (called “independent application”). Definition of antagonistic, additive, and synergistic apoptosis is described in “Statistical analysis.”
Next, we aimed to estimate the effect of the different application schedules of both drugs on a number of primary leukemia cells. Unfortunately, these cells allow only one single short-time experiment in vitro. To estimate how efficient the 2 drugs might have been if they had been given one after the other, independently from each other, independent apoptosis induction was calculated out of the data obtained by single-agent stimulation using the equation by Webb (Figure 2C, white and gray bars; details of calculations in “Statistical analysis”). This equation allows the most precise calculation on limited numbers of experiments21 (Figure 2C white and gray bars; details of calculations in “Statistical analysis”). For comparison, apoptosis induction by the drug combination is depicted as black dots in Figure 2C. In 11% of samples, apoptosis induction was increased by simultaneous application of doxorubicin and VCR compared with independent application yielding synergistic apoptosis. In contrast, simultaneous application of doxorubicin and VCR was less effective than independent application, yielding antagonistic apoptosis: When both drugs were given at peak plasma concentration, doxorubicin inhibited VCR-induced apoptosis in 34% (12 of 35) of samples, which increased to 54% (19 of 35) of samples when lower concentrations of doxorubicin were included (data not shown). Within the small cohort, no correlation of inhibition of VCR-induced apoptosis by doxorubicin with genetic alterations could be detected (data not shown).
In summary, doxorubicin frequently and markedly inhibited VCR-induced apoptosis in primary tumor cells from children. Importantly, doxorubicin exerted its antiapoptotic effect on leukemia cells obtained from those children who received simultaneous application of doxorubicin and VCR within induction therapy of the ongoing polychemotherapy trial.
Sequence dependency of the antagonism in vitro and in vivo
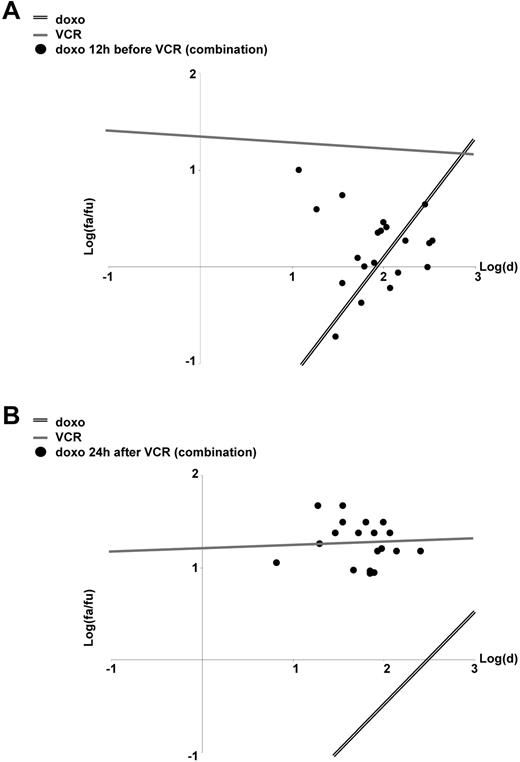
When 2 or more chemotherapeutic drugs are combined, sequence-dependent effects were revealed for a number of drug combinations both in vitro as well as in clinical studies.8,10,12-16 Therefore, we studied whether the antagonism between doxorubicin and VCR might be sequence dependent. Indeed, when VCR was given first, 1 day before doxorubicin, VCR induced significant apoptosis. In contrast, when doxorubicin was given first or together with VCR, doxorubicin markedly inhibited apoptosis induction by VCR (Figures 1B,3). At least in part, doxorubicin reduced VCR-induced apoptosis, even when given after VCR (supplemental Figure 4E). Thus, the efficiency of the combinatory treatment of doxorubicin and VCR depends on the sequence of application and whether doxorubicin encounters active VCR.
Sequence-dependent effects of the drug combination doxorubicin/VCR. (A-B) CEM cells were stimulated and analyzed as in Figure 1B, but now doxorubicin was applied 12 hours before VCR (A) or 24 hours after VCR (B).
Sequence-dependent effects of the drug combination doxorubicin/VCR. (A-B) CEM cells were stimulated and analyzed as in Figure 1B, but now doxorubicin was applied 12 hours before VCR (A) or 24 hours after VCR (B).
Taken together, doxorubicin inhibited apoptosis induction by VCR depending on the application schedule. These data add the clinical routine combination of anthracyclines and Vinca alkaloids to the drug combinations with sequence-dependent anti–tumor effects.
Signaling mechanism of VCR and inhibition by doxorubicin
To characterize underlying signaling mechanisms responsible for the negative drug interaction, doxorubicin and VCR were studied in parallel on CEM and JURKAT leukemia cell lines.
First, we aimed to characterize the effects of doxorubicin on VCR-induced apoptosis signaling events. The apoptosis signaling cascade activated by VCR is not completely characterized, but it involves incomplete spindle formation, cell-cycle arrest, activation of p53 and NF-κB, phosphorylation of antiapoptotic Bcl-2 members, and initiation of the downstream intrinsic apoptosis signaling cascade, among others.23 In a candidate approach, we analyzed the expression levels and functions of putative players.
VCR activates the intrinsic apoptosis signaling cascade. The Bcl-2 and IAP-member families contain important antagonists of this signal transduction. Their expression levels remained unchanged by treatment with doxorubicin, VCR, or the combination of both (supplemental Figure 2A). During VCR-induced apoptosis, antiapoptotic Bcl-2 and Bcl-xL become inactivated by phosphorylation.23 Apoptosis induction by VCR alone highly depended on phosphorylation of antiapoptotic Bcl-2 members, as overexpression of phosphorylation-deficient mutants of Bcl-2 and Bcl-xL markedly reduced apoptosis induction by VCR24,25 (supplemental Figure 2B). For the combined application of doxorubicin and VCR, the phosphorylation of Bcl-2 and Bcl-xL by VCR was markedly inhibited by doxorubicin (Figure 4A). Thus, failure to phosphorylate and inactivate antiapoptotic Bcl-2 and Bcl-xL was identified as the most proximal signaling step within the VCR-induced apoptotic signaling cascade, which was inhibited by doxorubicin. After lack of phosphorylation and inactivation of Bcl-2 and Bcl-xL, all downstream cell death signaling steps, which were rapidly activated on VCR-induced apoptosis, were inhibited by doxorubicin, including loss of mitochondrial membrane potential, release of cytochrome c, cleavage of caspases, and cleavage of PARP (Figure 4B).
Signaling mechanism of VCR and inhibition by doxorubicin. (A) Western blot of total cellular protein was performed on CEM cells stimulated with doxorubicin and VCR as in Figure 1A. GAPDH served as a loading control. (B) CEM cells were stimulated with doxorubicin and VCR. Loss of mitochondrial membrane potential (left panel) and cytochrome c release (right panel) was measured by FACScan. Caspase cleavage was detected by Western blot (bottom panel). *P < .05 (ANOVA), comparing stimulation with VCR alone with doxorubicin alone or combined stimulation with doxorubicin + VCR. (C) CEM cells were treated with 2,3-DCPE (10μM, left panel) or the phosphatase inhibitor okadaic acid (okadaic, 0.03 ng/mL, right panel) for 8 hours, followed by doxorubicin together with VCR for another 48 hours as indicated. Western blot of total cellular protein was performed after 56 hours. *P < .05 (ANOVA). NS indicates not significant. The concentrations of doxorubicin and VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A. Casp indicates caspase; co, unstimulated control cells; cl., cleaved; d, doxorubicin; p, phosphorylated; h, hour; and V, VCR.
Signaling mechanism of VCR and inhibition by doxorubicin. (A) Western blot of total cellular protein was performed on CEM cells stimulated with doxorubicin and VCR as in Figure 1A. GAPDH served as a loading control. (B) CEM cells were stimulated with doxorubicin and VCR. Loss of mitochondrial membrane potential (left panel) and cytochrome c release (right panel) was measured by FACScan. Caspase cleavage was detected by Western blot (bottom panel). *P < .05 (ANOVA), comparing stimulation with VCR alone with doxorubicin alone or combined stimulation with doxorubicin + VCR. (C) CEM cells were treated with 2,3-DCPE (10μM, left panel) or the phosphatase inhibitor okadaic acid (okadaic, 0.03 ng/mL, right panel) for 8 hours, followed by doxorubicin together with VCR for another 48 hours as indicated. Western blot of total cellular protein was performed after 56 hours. *P < .05 (ANOVA). NS indicates not significant. The concentrations of doxorubicin and VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A. Casp indicates caspase; co, unstimulated control cells; cl., cleaved; d, doxorubicin; p, phosphorylated; h, hour; and V, VCR.
To study the role of Bcl-2 members in apoptosis inhibition in more detail, DCPE was added, which is known to down-regulate Bcl-2 family members by as yet unknown mechanisms.26 DCPE reduced the expression levels of anti–apoptotic Bcl-2 and Bcl-xL as shown by Western blot (Figure 4C). Thereby, DCPE enabled to override the inhibitory effect of doxorubicin on VCR-mediated apoptosis (Figure 4C). Treatment with 2 phosphatase inhibitors, calphostin c and okadaic acid, caused phosphorylation of Bcl-2 and Bcl-xL independently from VCR by blocking dephosphorylation (Western blots in Figure 4C; and data not shown). Calphostin c and okadaic acid restored VCR-induced apoptosis in the presence of doxorubicin, supporting an important role for the stabilization of antiapoptotic Bcl-2 members in doxorubicin-induced inhibition of apoptosis (Figure 4C; and data not shown).
Taken together, these results show that doxorubicin stabilized anti–apoptotic Bcl-2 family members by preventing their phosphorylation, which was reversed by treatment with DCPE, calphostin c, and okadaic acid. Doxorubicin inhibited VCR-induced Bcl-2 member phosphorylation and thereby disabled the downstream intrinsic apoptosis signaling cascade of VCR (Figure 6E).
Signaling mechanism of doxorubicin and impact on VCR-induced apoptosis
Next, we searched for signaling molecules that mediate the antiapoptotic function of doxorubicin. In tumor cells, anthracyclines are known to intercalate into DNA, induce formation of free radicals, and inhibit topoisomerase II among other actions.27 Anthracyclines induce DNA damage, activate ataxia teleangiectasia mutated kinase, induce p53, and lead to cell-cycle arrest or apoptosis.27,28
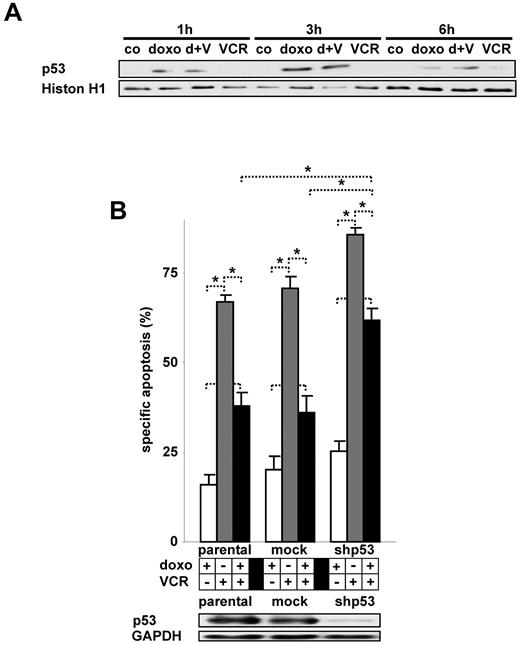
The transcription factor p53 is activated by DNA-damage and signals pleiotrophic effects, such as DNA repair, cell-cycle arrest, and cell death.29 In our 2 cell lines investigated, p53 is present and doxorubicin induced the accumulation of nuclear p53 both in the presence and absence of VCR (Figure 5A; data not shown). The down-regulation of p53 protein levels restored VCR-induced apoptosis in the presence of doxorubicin, indicating an important role for p53 in this process (Figure 5B).
Activation of p53 by doxorubicin and its impact on VCR-induced apoptosis. (A) Nuclear extracts of CEM cells simultaneously stimulated with doxorubicin and VCR as indicated were analyzed by Western blot. Histone H1 served as a loading control. (B) Parental CEM cells stably transfected with shRNA targeting p53 (shp53) or a control mock shRNA sequence were stimulated with doxorubicin and VCR simultaneously. *P < .05 (ANOVA). NS indicates not significant. Western blot was performed of total cellular protein. The concentrations of doxorubicin and VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A. d indicates doxo; V, VCR; h, hour; and co, unstimulated control cells.
Activation of p53 by doxorubicin and its impact on VCR-induced apoptosis. (A) Nuclear extracts of CEM cells simultaneously stimulated with doxorubicin and VCR as indicated were analyzed by Western blot. Histone H1 served as a loading control. (B) Parental CEM cells stably transfected with shRNA targeting p53 (shp53) or a control mock shRNA sequence were stimulated with doxorubicin and VCR simultaneously. *P < .05 (ANOVA). NS indicates not significant. Western blot was performed of total cellular protein. The concentrations of doxorubicin and VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A. d indicates doxo; V, VCR; h, hour; and co, unstimulated control cells.
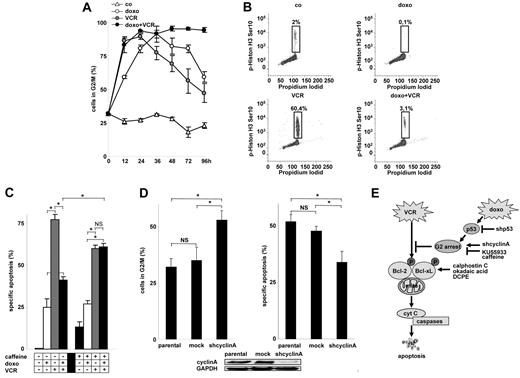
Activated p53 induces cell-cycle arrest, which might participate in apoptosis inhibition. Both VCR and doxorubicin caused a marked accumulation of cells in G2/M (Figure 6A; supplemental Figure 3A). Further cell-cycle discrimination revealed that doxorubicin arrested the cell cycle in G2, whereas VCR arrested the cell cycle in M (Figure 6B). No signs of cellular senescence were detected (data not shown). When drugs were combined, cell-cycle arrest was enhanced and > 80% of the cells were arrested in the G2 for the entire observation period available in cell culture (Figure 6A; and data not shown). Cell-cycle arrest in G2 by doxorubicin was reduced in p53 knockdown cells (supplemental Figure 3B).
Arrest of the cell cycle by doxorubicin and its impact on VCR-induced apoptosis. (A-B) Cell-cycle analysis was performed using propidium iodide staining of DNA in CEM cells (A). Corresponding cell-cycle histograms are presented in supplemental Figure 3A. To discriminate between G2 and M arrest, double staining for phospho-histone H3 (Ser10) and propidium iodide was performed after 24 hours (B). co indicates unstimulated control cells. (C) CEM cells were preincubated with caffeine (300 μg/mL) for 8 hours, followed by doxorubicin together with VCR for 48 hours. *P < .05 (ANOVA). NS indicates not significant. (D) CEM cells were stably transfected with a shRNA targeting cyclin A (shcyclin A) or a control mock sequence and were analyzed for cell-cycle distribution of spontaneously growing cells (left panel) or for apoptosis induction by VCR (3 ng/mL) after 48 hours (right panel). *P < .05 (ANOVA). NS indicates not significant. (E) Scheme summarizing the data presented in Figures 4 to 6: Doxorubicin-mediated activation of p53 and G2 arrest inhibits VCR-induced cell death, abrogating VCR-induced phosphorylation of Bcl-2 family members, the distal apoptosis signaling pathway, and cell death. The concentrations of doxorubicin and VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A.
Arrest of the cell cycle by doxorubicin and its impact on VCR-induced apoptosis. (A-B) Cell-cycle analysis was performed using propidium iodide staining of DNA in CEM cells (A). Corresponding cell-cycle histograms are presented in supplemental Figure 3A. To discriminate between G2 and M arrest, double staining for phospho-histone H3 (Ser10) and propidium iodide was performed after 24 hours (B). co indicates unstimulated control cells. (C) CEM cells were preincubated with caffeine (300 μg/mL) for 8 hours, followed by doxorubicin together with VCR for 48 hours. *P < .05 (ANOVA). NS indicates not significant. (D) CEM cells were stably transfected with a shRNA targeting cyclin A (shcyclin A) or a control mock sequence and were analyzed for cell-cycle distribution of spontaneously growing cells (left panel) or for apoptosis induction by VCR (3 ng/mL) after 48 hours (right panel). *P < .05 (ANOVA). NS indicates not significant. (E) Scheme summarizing the data presented in Figures 4 to 6: Doxorubicin-mediated activation of p53 and G2 arrest inhibits VCR-induced cell death, abrogating VCR-induced phosphorylation of Bcl-2 family members, the distal apoptosis signaling pathway, and cell death. The concentrations of doxorubicin and VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A.
To prevent cell-cycle arrest induced by doxorubicin and VCR, cells were pretreated with caffeine or KU-55933, an inhibitor of ataxia teleangiectasia mutated kinase. Both compounds alleviated the block in the presence of both doxorubicin and VCR (supplemental Figure 3C; data not shown) and sensitized the cells for VCR-induced apoptosis (Figure 6C; and data not shown).
To arrest the cell cycle on a molecular level, cyclin A was knocked down by RNA interference, which induced a major fraction of cells in G2 (Figure 6D). Knockdown of cyclin A and concomitant cell-cycle arrest significantly inhibited VCR-induced apoptosis (Figure 6D) proving that cell-cycle arrest disabled VCR-induced apoptosis.
Taken together, these results show that p53 and cell-cycle arrest mediate the antiapoptotic function of doxorubicin. Doxorubicin inhibited VCR-induced apoptotic signaling upstream of Bcl-2/Bcl-xL. Lack of p53, addition of 2 cell-cycle stimulators, caffeine or KU55933, or addition of agents antagonizing Bcl-2/Bcl-xL, such as calphostin c, okadaic acid, and DCPE, alleviated the antiapoptotic function of doxorubicin toward VCR-induced apoptosis, whereas knockdown of cyclin A inhibited VCR-induced apoptosis similarly to doxorubicin as illustrated in a schematic chart (Figure 6E).
Cell-cycle arrest–based antagonistic interaction between irradiation and VCR
So far, our mechanistic studies revealed that cell-cycle arrest mediated doxorubicin-induced inhibition of VCR-induced apoptosis. Based on these new mechanistic insights, we hypothesized that further cell-cycle arresting drugs and stimuli would inhibit VCR-induced apoptosis similarly to doxorubicin.
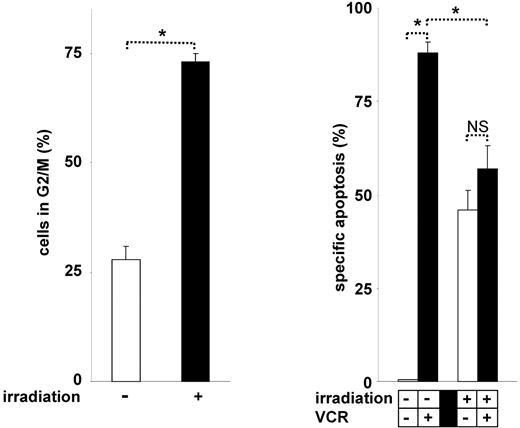
Indeed, irradiation induced cell-cycle arrest in G230,31 and significantly inhibited VCR-induced cell death (Figure 7). Similarly to the phenotype observed in G2 arrested cells, cell-cycle arrest in G0 or G1 by dexamethasone, serum starvation, or L-mimosine hampered apoptosis induction by VCR (data not shown). These data show that VCR is generally unable to induce cell death in cell-cycle–arrested tumor cells and that VCR requires active cell cycling for effective induction of apoptosis. Thus, our new understanding of the mechanisms of VCR-induced apoptosis enabled the identification of numerous antagonizing stimuli.
Cell-cycle arrest–based antagonistic interaction between irradiation and VCR. CEM cells were irradiated with 6 Gy for 24 hours. Cell-cycle analysis was performed (left panel), and cells were stimulated with VCR for another 48 hours (right panel). *P < .05 (ANOVA). NS indicates not significant. The concentration of VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A.
Cell-cycle arrest–based antagonistic interaction between irradiation and VCR. CEM cells were irradiated with 6 Gy for 24 hours. Cell-cycle analysis was performed (left panel), and cells were stimulated with VCR for another 48 hours (right panel). *P < .05 (ANOVA). NS indicates not significant. The concentration of VCR, measurement of apoptosis, presentation of data, and statistical analysis were performed as described in Figure 1A.
Taken together, we have identified cell-cycle arrest as a new general mechanism responsible for sequence-dependent effects of drug combinations with VCR: If VCR is given first and activates the proapoptotic signaling cascade before induction of cell-cycle arrest by any second stimulus, VCR potently induces apoptosis. If a cell-cycle arresting, cytostatic stimulus is given shortly before VCR, the proapoptotic efficacy of VCR is markedly reduced.
Discussion
In several different polychemotherapy protocols for hematopoietic tumors, anthracyclines and Vinca alkaloids are given on the same day. Our data show that the anti–tumor effect of this drug combination might be enhanced if both drugs are given on different days.
In vitro, the drug combination was found to be highly sequence-dependent both in tumor cell lines and in primary, patient-derived tumor cells. Sequence dependency was also found in an in vivo trial. Using complex signaling studies and knockdown strategies, we delineate the underlying signaling mechanism for antagonistic apoptosis: Doxorubicin activates p53, p53 induces cell-cycle arrest, and cell-cycle arrest inhibits the downstream apoptosis signaling pathway otherwise activated by VCR. Characterization of this signaling mechanism enabled to identify further antagonistic drug combinations (eg, irradiation and VCR).
Back in the 1970s, clinicians decided to combine anthracyclines and Vinca alkaloids during polychemotherapy based on few (< 10) animal experiments performed on rat and mouse tumor cells.6,7 In these animal trials, high concentrations of doxorubicin potentiated VCR-induced anti–tumor efficiency. Regarding the combination of doxorubicin and VCR; and as far as we know, no systematic evaluation of application schedules was ever performed on human tumor cells either in vitro or in small animal models in vivo or in clinical trials in patients.
In contrast, in our study, we used human leukemia cell lines in vitro and in vivo and primary tumor cells of children with acute leukemia ex vivo and found that anthracyclines markedly inhibited Vinca alkaloid-induced apoptosis. Here, independent application of doxorubicin and VCR was more effective against most tumor cells than combined application in vitro. To study the biologic effects and signaling mechanisms of chemotherapeutic drugs, we used drug concentrations in vitro, which are achieved in the plasma of patients during anti–tumor therapy in vivo (peak plasma concentration = ppc as maximum).32 The difference between the previous data and our data might rely on factors, such as interspecies differences, drug concentrations used, different read-outs, and the small number of cell lines tested in those former studies.
It will be interesting to evaluate whether the independent application of anthracyclines and Vinca alkaloids will increase treatment efficiency in cancer patients, especially as doxorubicin and VCR showed antagonistic effects in the primary tumor cells of one-third of children treated with this combination.
The newly discovered antiapoptotic function of doxorubicin is in line with our previous data showing that TRAIL, a chemotherapeutic drug currently in phase 1 and 2 clinical trials, displays antiapoptotic and even pro-proliferative features in apoptosis-resistant tumor cells.18-20
There is a long-lasting conceptual discussion of whether cytotoxic and cytostatic drugs should be combined.33 The term “cytostatic drugs” has been used for drugs that act against tumor cells by inducing cell-cycle arrest. In our study, anthracyclines arrested the cell cycle and behaved like cytostatic drugs in the cell lines investigated. On the other hand, several chemotherapeutic drugs require active cell cycling for induction of apoptosis and cell-cycle arrest reduces or abrogates the anti–tumor efficiency of these drugs.16,33 In our experiments, Vinca alkaloids acted like drugs depending on active cell cycling. Our data allow the hypothesis that cytostatic drugs and drugs depending on active cell cycling might not be combined, as they exert optimal anti–tumor efficiency if given independently.
Vinca alkaloids are not the only class of chemotherapeutic drugs that depends on active cell cycling.16 More studies are required to identify those drugs, which share the molecular mechanism of Vinca alkaloids and should therefore not be combined with cytostatic drugs.
p53 was shown by others34 and by us19 to predominantly act as a proapoptotic mediator. In contrast to its mainly proapoptotic functions, in our study p53 acted as an apoptosis inhibitor toward VCR-induced apoptosis. Although cell lines contained different p53 conformations and mutations, no correlation was found between mutations of p53 and interaction between doxorubicin and VCR.22
The present work clearly underlines the significance to reevaluate the inhibitory effect within the multiple known functions of p53.35 Because many chemotherapeutic drugs activate p53, the new anti–apoptotic effect might not be restricted to anthracyclines and irradiation, but might involve additional established chemotherapeutic drugs that activate p53. Our data allow the hypothesis that further stimuli of p53 will disable VCR-induced apoptosis.
It is well known that (1) numerous chemotherapeutic drugs activate p53, (2) p53 can induce cell-cycle arrest in tumor cells, and (3) certain chemotherapeutic drugs need active cell cycling for the induction of cell death.16,19,23,29,31 The new antiapoptotic function of anthracyclines, although clinically and empirically surprising, is not surprising once the underlying molecular mechanisms are considered.
Our data represent the first characterization of a molecular mechanism responsible for sequence-dependent anti–tumor effects of chemotherapeutic drugs in routine clinical use. Our data clearly reinforce the need for the detailed mechanistic understanding of signaling pathways and pathway cross-talks. “Targeted therapies” represent a promising concept to optimize anti–tumor therapy based on the mechanistic understanding of target proteins and cellular signaling. In parallel, our data encourage to widen the concept and search for “targeted drug combinations” in which the positive or negative interaction of drugs is characterized on a molecular level. These new mechanistic insights will enable the design of more effective polychemotherapy protocols to treat hematopoietic malignancies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank T. Chou for all recommendations of appropriate test modalities and analysis of drug interaction, and L. Mura from Helmholtz Zentrum München and R. Meilbeck from the Dr von Haunersches Kinderspital for skilled technical work. Phosphorylation- deficient mutants of Bcl-2 were a kind gift from the laboratory of S. Korsmeyer.
This work was supported by Wilhelm Sander-Stiftung, Deutsche Jose Carreras Leukämie Stiftung, Dr Helmut Legerlotz Stiftung, and Bettina Bräu Stiftung (all to I.J.).
Authorship
Contribution: H.E., D.S., C.M., and F.W. performed experiments; S.H. provided phosphorylation-deficient Bcl-xL expression plasmids; U.G. and M.N. provided primary patient samples; H.E. and I.J. designed the research, provided administrative support, analyzed and interpreted the data, prepared the figures, and wrote the paper; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irmela Jeremias, Helmholtz Center Munich, German Research Center for Environmental Health, Marchioninistrasse 25, D-81377 München, Germany; e-mail: irmela.jeremias@helmholtz-muenchen.de.