Abstract
Iron is an essential component of heme and hemoglobin, and therefore restriction of iron availability directly limits erythropoiesis. In the present study, we report a defect in iron absorption that results in iron-deficiency anemia, as revealed by an N-ethyl-N-nitrosourea–induced mouse phenotype called sublytic. Homozygous sublytic mice develop hypochromic microcytic anemia with reduced osmotic fragility of RBCs. The sublytic phenotype stems from impaired gastrointestinal iron absorption caused by a point mutation of the gastric hydrogen-potassium ATPase α subunit encoded by Atp4a, which results in achlorhydria. The anemia of sublytic homozygotes can be corrected by feeding with a high-iron diet or by parenteral injection of iron dextran; rescue can also be achieved by providing acidified drinking water to sublytic homozygotes. These findings establish the necessity of the gastric proton pump for iron absorption and effective erythropoiesis.
Introduction
Body iron content is tightly regulated. Because no efficient pathway exists for iron excretion, body iron is regulated primarily at the level of dietary iron absorption.1,2 Iron is absorbed by intestinal enterocytes in the duodenum, where the acidic environment is essential to permit ferric iron, the predominant form of dietary non-heme iron, to remain soluble for chelation or reduction to ferrous iron. Gastric acid also provides the iron transporter DMT1 with protons required for the cotransport of ferrous iron across enterocyte membranes.
The acidification of the stomach (pH 2-3) is achieved by hydrogen potassium ATPase (H+,K+-ATPase) on parietal cells, which catalyzes the exchange of luminal potassium ions for cytoplasmic protons. This enzyme consists of an α-subunit that carries out the catalytic and transport functions of the enzyme,3 and a β-subunit that protects the enzyme from degradation and is necessary for trafficking to the plasma membrane.4 The H+,K+-ATPase mediates the final step in acid secretion, and therefore serves as a target in therapies for acid-related diseases.5 Proton pump inhibitors (PPIs) directly bind to and inactivate H+,K+-ATPase, raising the median gastric pH to > 3.6.6,7 Interestingly, despite their widespread use, pharmacologic suppression of gastric acid with PPIs is not associated with an increased risk of iron-deficiency anemia.6,8,9 Therefore, the prevailing view is that gastric acid facilitates the absorption of dietary iron but is not indispensable for this purpose under normal conditions.9,10
In addition, no iron-deficiency phenotype has been described in rodent models in which the genes encoding the 2 subunits of the H+,K+-ATPase (Atp4a and Atp4b) were knocked out.3,4 In these animals, the absence of functional H+,K+-ATPase leads to achlorhydria and a neutral gastric pH. Despite being fed a normal diet containing only non-heme iron, iron-deficiency anemia was not reported.3,4 Therefore, although the importance of gastric acidity for iron absorption is well accepted, a clear link between H+,K+-ATPase inactivation and iron-deficiency anemia has not been established.
In the current study, we describe the N-ethyl-N-nitrosourea–induced mutant phenotype sublytic, so named for the relative resistance of its RBCs to ammonium chloride lysis. Sublytic mice developed hypochromic microcytic anemia with iron deficiency, which is manifested by severely reduced iron stores. The causal mutation is a T → C transition in Atp4a, encoding the α subunit of the gastric H+,K+-ATPase. The underlying cause of anemia in sublytic mice is poor iron absorption that is likely attributable to an abnormally high gastric pH. Therefore, by promoting iron absorption, H+,K+-ATPase permits effective erythropoiesis in mice.
Methods
Mice
C57BL/6J strain animals were used for mutagenesis, as described previously.11 Atp4a−/− mice (generated on a hybrid background of 129/SvJ and Black Swiss strains, and later purified by multigeneration backcross to the C57BL/6J background) were obtained from Dr Gary E. Shull (University of Cincinnati College of Medicine, Cincinnati, OH). All mouse experiments were performed in accordance with institutional regulations governing animal care and use and were approved by the Scripps Research Institute Institutional Animal Care and Use Committee. Animals were maintained under standard housing conditions and fed with irradiated normal rodent chow (no. 5058 PicoLab Mouse Diet 20, LabDiet; PMI Nutrition International), except when iron-supplemented (obtained from Teklad, Harlan, TD03439) or iron-deficiency food was used (TD80396, Teklad, Harlan). All studies were conducted in conformity with University of California-San Diego International Law and Regulation Laboratory (ILAR) regulations. Euthanasia, when performed, was by isoflurane inhalant overdose. The sublytic strain is described further at http://mutagenetix.utsouthwestern.edu.
Hematology analysis
Whole blood was collected from retro-orbital cavity into potassium EDTA–containing Microvette 100 tubes (Sarstedt) and analyzed on a Scil Vet abc automatic hematology analyzer (scil animal care). The reticulocyte count was analyzed with a Retic-COUNT (Thiazole Orange) Reagent (BD Biosciences). Blood smears were stained with Wright-Giemsa stain. The serum erythropoietin level was quantified with Mouse/Rat Erythropoietin Quantikine ELISA Kit (R&D Systems).
Osmotic fragility test
Fresh blood was collected from 6-week-old mice into EDTA-containing Microvette 100 tubes, and the test was performed within 2 hours of collection. One microliter of whole blood or an equal amount of RBCs was mixed with 200 μL of NaCl solution with a gradient ranging from 0.10%-0.85%, respectively, and incubated at room temperature for 20 minutes. Cells were then centrifuged and the supernatant's absorbance was measured at 540 nm. The percentage hemolysis is calculated for each solution and plotted against NaCl concentrations. The degree of lysis in 0.10% NaCl was considered to be 100% and for 0.85% NaCl, 0%.
BM transplantation and immunostaining of BM and spleen cells
Femurs from sublytic (on a pure, CD45.2+, C57BL/6J background) or CD45.1+ congenic mice were dissected and the BM was flushed into sterile PBS. 2 × 106 cells were injected intravenously into recipient mice (CD45.1+ or sublytic mice), which were irradiated with 1000 Rad. Recipients were kept on antibiotic-treated water for 3 weeks, and the phenotypes of chimera mice were analyzed 8 weeks after reconstitution. Chimerism was assessed by staining splenocytes with the congenic marker CD45.1.
To assess the population of erythrocyte precursors, BM and spleen cells were incubated with Abs to mouse TER119 (catalog no. 557909; BD Biosciences) and CD71 (catalog no. ab22393; Abcam), respectively, as described previously.12 Results were acquired on a FACSCalibur flow cytometer (BD Biosciences) with CellQuest Pro Version 6.0 software and analyzed using FlowJo Version 7.6 software.
Measurement of serum iron levels
Blood was collected from mice by retro-orbital bleeding. A colorimetric assay was used to assess serum iron levels as described previously.13 Briefly, iron standards (Thermo Scientific) in the concentration range from 31.25-500 μg/dL were assayed at the same time. Serum or standard was mixed with an acid-precipitating/reducing solution (0.6M trichloroacetic acid, 0.4M sodium thioglycollate, and 2M HCl). The samples were then centrifuged and the supernatant was removed from each sample and added to the chromogen bathophenanthroline (1.5M sodium acetate and 0.5mM bathophenanthroline disulfonic salt). The optical density of each sample was measured at 535 nm, and the serum iron concentration of each sample was calculated using the iron standard curve.
Gastric iron absorption
Iron absorption was measured by total body counting using a modification of a previously described method.14 In brief, radiolabeled iron in the form of 59FeCl3 was diluted in 0.2 mL of 1M ascorbic acid in PBS containing 6 μg of unlabeled elemental iron (as FeCl3). Mice were fasted overnight before gavage but allowed water ad libitum. The total volume introduced was 0.2 mL/mouse (2μCi 59Fe). Animals were then fasted for 7 more hours, and then allowed food overnight. Approximately 24 hours after gavage, the mice were euthanized and the gastrointestinal tracts were removed. The remaining radioactivity in the carcass was counted using a LIVE-1 whole-animal γ counter (Technical Associates). The percentage of absorption was defined as the amount of radioactivity in the whole animal minus the gastrointestinal tract divided by the amount instilled by gavage.
Histology and tissue iron staining
Spleen sections from wild-type and sublytic mice were stained with H&E. Iron staining was performed with standard Prussian blue staining procedure. Histology images were acquired using an Olympus BH2 microscope and an Olympus Magnafire digital image capture system and AxioVision Release 4.8.1 software or with a Leica SCN400 digital slide scanner using SlidePath DIH Version 3.0 software.
Iron-repletion studies
The high-iron diet (#TD.03439) was made by adding 2% carbonyl iron to standard rodent diet 7012 (Teklad, Harlan), which contains iron at a concentration of 284 ppm. Animals were maintained on the diet for 4 weeks. Alternatively, 1 mg of iron-dextran (D8517; Sigma-Aldrich) was injected IP to sublytic mice and wild-type controls, and the RBC indices were evaluated 2 weeks later.
Genetic mapping
Genetic mapping was accomplished by bulk segregation analysis as described previously.15 In brief, sublytic homozygous mice were first outcrossed with wild-type C57BL/10J mice, and their offspring F1 mice were then intercrossed to generate F2 animals for mapping purposes. The allele frequency was measured at each informative locus in pools of DNA from phenotypically affected and nonaffected F2 mice. The mapping panel consists of 127 single nucleotide polymorphisms (SNPs) validated by capillary sequencing: 124 spaced at ∼ 20-Mb intervals across the 19 autosomes and 3 markers on the X chromosome. Each SNP was amplified by PCR from the 2 pooled F2 DNA samples. DNA sequencing trace peak heights were used to interpolate C57BL/6J (B6) and C57BL/10J (B10) allele frequencies at each SNP site in the F2 test samples from standard curves of normalized peak height versus allele frequency, generated using DNA samples containing known ratios of B6:B10 DNA. The estimated B6 and B10 allele numbers for each SNP site were determined as the estimated allele percentage × N, and were used to calculate P values separately for mice with mutant and normal phenotypes on the basis of the χ2 distribution and the expected frequencies of B6 and B10 alleles at each variant site. The probabilities for the mutant phenotype sample and the normal phenotype sample were combined after calculation using the Fisher method of combining P values with the formula χ2 = −2[ln(p1) + ln(p2)]. The P value for χ2, Pcomposite, was determined from a χ2 distribution with 4 degrees of freedom. Scores for linkage were calculated as −log10(P) separately for the mutant phenotype pool, the normal phenotype pool, and for all mice combined.
Whole-genome sequencing
Whole-genome sequencing was performed as described previously.16 Briefly, a tail sample from a sublytic mouse was digested overnight at 55°C in lysis buffer (100mM Tris, 0.2% SDS, 5mM EDTA, 200mM NaCl, and 2 mg/mL of proteinase K). The digested DNA was subjected to phenol and chloroform extraction, followed by precipitation with 3M sodium acetate (pH 5.5) and 100% isopropanol. The DNA was then washed twice with 70% ethanol, and resuspended in nuclease-free water for preparation of the SOLiD3+ library.
Raw SOLiD sequencing data were processed through a pipeline that includes data capture, alignment to the reference sequence (National Center for Biotechnology Information reference assembly build 37) using a Linux cluster computer, filtration to remove false positives, and automated validation sequencing of the potential mutations.
Quantitative RT-PCR
Liver, stomach, or small intestine RNA was extracted using TRIzol reagent (Invitrogen), and 1 μg of total RNA was reverse transcribed using RETROscript First Strand Synthesis (Applied Biosystems) with the random decamers primer or the oligo(dT)18 primer, respectively. Quantitative PCR was carried out on a 7300 Real Time PCR system (Applied Biosystems). The primers used were: Hamp1: 5′ TTGCGATACCAATGCAGAAGA 3′ and 5′ GATGTGGCTCTAGGCTATGTT 3′; 18S: 5′ ACTTTTGGGGCCTTCGTGTC 3′ and 5′ GCCCAGAGACTCATTTCTTCTTG 3′; Atp4a: 5′ CATTGCCTTTGCCATCCAGG 3′ and 5′ CTGGTAGTAGCCAAAACAGCC 3′; Slc11a2: 5′ AGTATGTCACCGTCAGTATCCC 3′ and 5′ CTGAGCCAATGACTTCCTGC 3′; and Hprt: 5′ CTGGTTAAGCAGTACAGCCCCAA 3′ and 5′ CAGGAGGTCCTTTTCACCAGC 3′. The results were expressed as the ratio of target transcript to that of housekeeping gene.
RBCs in vivo survival assay
N-hydroxysuccinimide-biotin (E-Z link; Thermo Scientific) was suspended in sterile saline at a concentration of 4 mg/mL, and then injected intravenously into sublytic and wild-type control mice, respectively, at a dose of 40 mg/kg body weight. Animals were first bled at 36 hours after injection, and then at 2- to 3-day intervals for the first 2 weeks, followed by 3- to 4-day intervals for the third and fourth weeks, and then twice more weekly. For each blood sampling, 5-10 μL of blood was taken from the retro-orbital cavity. Labeled cells were analyzed by FACS after staining with streptavidin PE. The percentage of biotinylated RBCs over time reflects the survival of RBCs from each genotype group.
Measurement of gastric pH
Sex-matched wild-type and sublytic mice (8 weeks old) were fasted for 2 hours before the experiment. Histamine HCl (H7250, Sigma-Aldrich) was suspended in PBS at a concentration of 400 μg/mL, and injected subcutaneously at a dose of 2 μg/g of body weight. After 45 minutes, the mice were euthanized and their stomachs were removed. Stomachs were minced into 2 mL of normal saline, insoluble material was pelleted by centrifugation, and the pH of the supernatant was measured.3
Statistical analysis
Statistical significance was evaluated using the unpaired Student t test, and differences with P < .05 were considered statistically significant. Prism Version 5 software (GraphPad) was used for statistical evaluation.
Results
Sublytic mice exhibit severe anemia
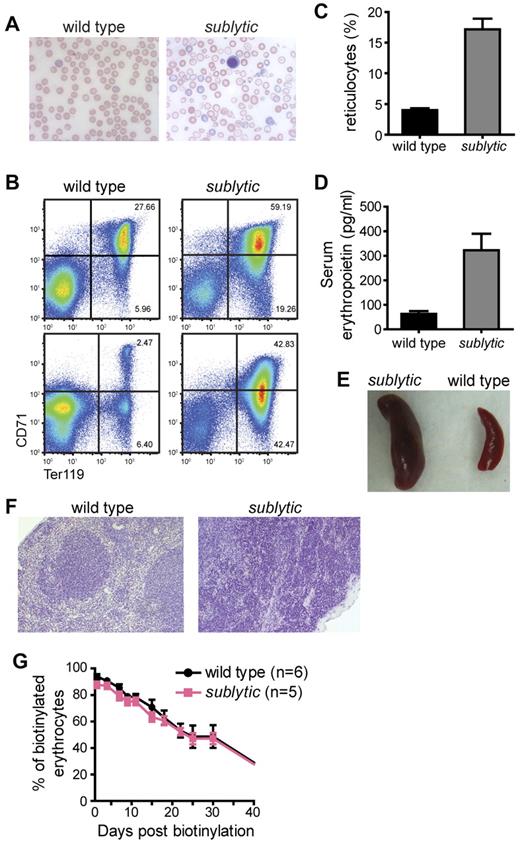
The recessive sublytic phenotype was identified in a genetic screen for hematologic abnormalities in N-ethyl-N-nitrosourea– mutagenized C57BL/6J mice. Sublytic homozygotes exhibited significantly reduced hematocrit and hemoglobin content (including both hemoglobin and mean corpuscular hemoglobin) relative to wild-type control mice (Table 1). Mean corpuscular volume (MCV) was also significantly smaller in sublytic compared with wild-type mice, and Wright-Giemsa staining of blood smears from sublytic mice revealed hypochromic RBCs with striking anisocytosis and poikilocytosis (Figure 1A). Leukocyte populations were normal in number and appearance. Sublytic homozygotes were grossly normal in appearance, body growth, and lifespan compared with wild-type littermates.
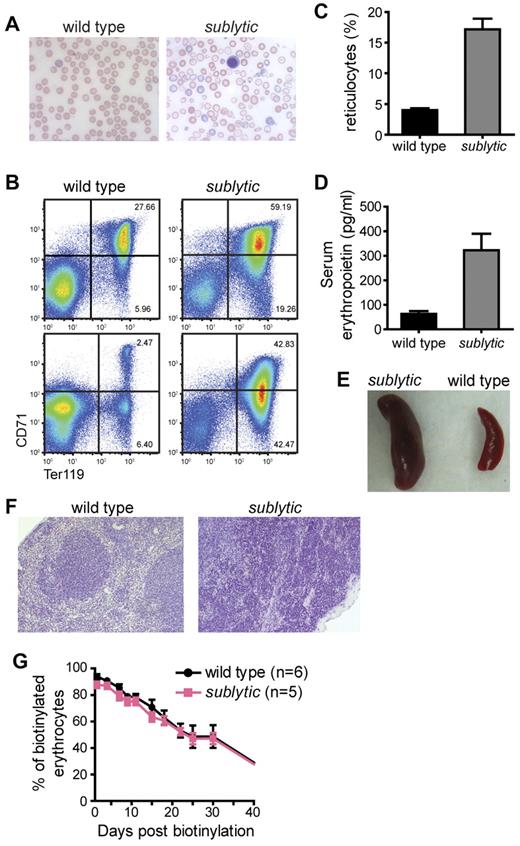
Sublytic mice develop severe anemia. (A) Morphology of RBCs from a wild-type and sublytic mouse. RBCs were examined after Wright-Giemsa staining, and photographed at a 63× magnification (oil) under light microscopy. (B) BM cells (top panels) and splenocytes (bottom panels) prepared from wild-type and sublytic mice were labeled with Ter119 and CD71 Abs and analyzed by flow cytometry. Values indicate the percentage of cells in each quadrant. (C) Reticulocytes were stained with thiazole orange and counted by flow cytometry. The percentage of reticulocytes in the blood is shown (n = 6 for both wild-type and sublytic mice). (D) Serum erythropoietin was measured by ELISA (n = 3 for both wild-type and sublytic mice). Data represent means ± SEM. (E) Whole spleens from a sublytic homozygous and a wild-type mouse at approximately 9 months of age. (F) Sections of spleens from wild-type and sublytic mice stained with H&E are shown at a 20× magnification. (G) In vivo RBC survival. RBCs were labeled with biotin in vivo by injecting mice with N-hydroxysuccinimide-biotin at 6 weeks of age. The survival of RBCs was followed by measuring the percentage of labeled erythrocytes by flow cytometry.
Sublytic mice develop severe anemia. (A) Morphology of RBCs from a wild-type and sublytic mouse. RBCs were examined after Wright-Giemsa staining, and photographed at a 63× magnification (oil) under light microscopy. (B) BM cells (top panels) and splenocytes (bottom panels) prepared from wild-type and sublytic mice were labeled with Ter119 and CD71 Abs and analyzed by flow cytometry. Values indicate the percentage of cells in each quadrant. (C) Reticulocytes were stained with thiazole orange and counted by flow cytometry. The percentage of reticulocytes in the blood is shown (n = 6 for both wild-type and sublytic mice). (D) Serum erythropoietin was measured by ELISA (n = 3 for both wild-type and sublytic mice). Data represent means ± SEM. (E) Whole spleens from a sublytic homozygous and a wild-type mouse at approximately 9 months of age. (F) Sections of spleens from wild-type and sublytic mice stained with H&E are shown at a 20× magnification. (G) In vivo RBC survival. RBCs were labeled with biotin in vivo by injecting mice with N-hydroxysuccinimide-biotin at 6 weeks of age. The survival of RBCs was followed by measuring the percentage of labeled erythrocytes by flow cytometry.
The severity of anemia in sublytic mice changed with age, but was observed equally between male and female mice. All analyses were performed with both male and female mice. At 4-6 weeks of age, sublytic mice exhibited highly elevated numbers of Ter119highCD71high/med erythroid progenitor cells in the BM and spleen, which were associated with reticulocytosis and elevation of serum erythropoietin (Figure 1B-D), and often with accompanying thrombocytosis (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The reticulocytosis was commensurate with the severity of anemia, suggesting that it was a compensatory response. In most cases, the increased erythropoiesis increased RBC numbers (supplemental Figure 2B), hemoglobin level (supplemental Figure 2C), and hematocrit, but not MCV, in sublytic homozygotes, albeit not to the levels observed in wild-type mice during the period from 4-6 weeks of age. After 8 weeks of age, RBC numbers, hemoglobin, and reticulocyte numbers approached wild-type levels and were maintained for several months thereafter (supplemental Figure 2A-C). Eventually, some sublytic mice again became severely anemic, as measured by RBC hemoglobin, RBC numbers, and serum iron (supplemental Figure 2A-D), and also developed splenomegaly (Figure 1E). Complete disruption of normal splenic architecture by extramedullary erythropoiesis was observed in the spleen (Figure 1F). The age at which sublytic homozygotes developed this severe anemia was variable, occurring at 10 months of age or much later (∼ 20 months). MCV was reduced in sublytic mice at all ages analyzed.
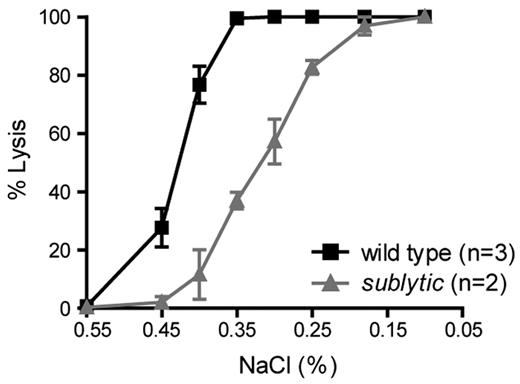
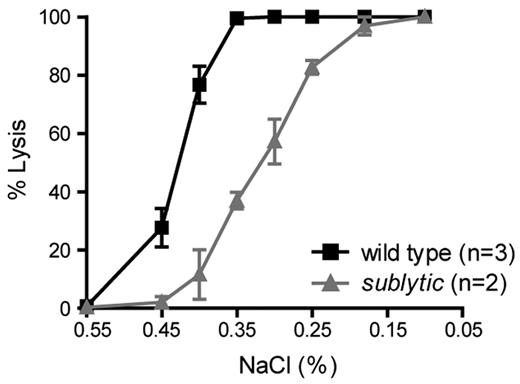
To assess the survival of RBCs, we followed the percentage of biotin-labeled erythrocytes in N-hydroxysuccinimide-biotin–injected mice by flow cytometry over a time course of 50 days. The erythrocyte half-life in 8-week-old sublytic mice was 24 days compared with 26 days in the controls (Figure 1G). We also observed that RBCs from sublytic mice exposed to hypotonic saline solutions ranging from 0.1%-0.85% NaCl showed diminished osmotic fragility compared with that of RBCs from wild-type mice (Figure 2).
Sublytic erythrocytes are resistant to osmotic lysis. One microliter of fresh blood was added to 0.2 mL of a series of hypotonic solutions ranging from 0.1%-0.85% NaCl. The percentage of lysed erythrocytes was calculated based on absorbance. The degree of lysis in 0.1% and 0.85% NaCl was considered to be 100% and 0%, respectively. Data represent the means ± SEM.
Sublytic erythrocytes are resistant to osmotic lysis. One microliter of fresh blood was added to 0.2 mL of a series of hypotonic solutions ranging from 0.1%-0.85% NaCl. The percentage of lysed erythrocytes was calculated based on absorbance. The degree of lysis in 0.1% and 0.85% NaCl was considered to be 100% and 0%, respectively. Data represent the means ± SEM.
Sublytic homozygotes develop severe iron deficiency due to impaired intestinal iron absorption
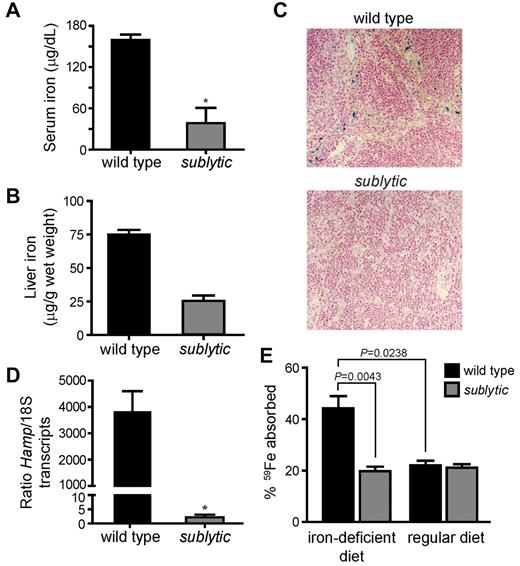
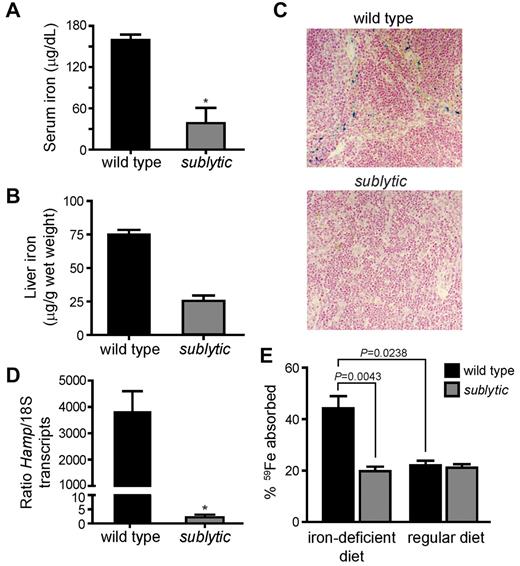
Because of the significantly reduced MCV observed in sublytic homozygotes, iron deficiency was suspected. We evaluated the iron status of sublytic mice by checking serum iron levels and iron stores. Indeed, sublytic mice showed significant attenua-tion of serum iron and depleted iron stores in the spleen and liver (Figure 3A-C).
Sublytic mice are iron deficient. (A) Serum iron levels (n = 5 for wild-type mice and n = 3 for sublytic mice, P = .0357). (B) Liver iron stores (n = 4 for wild-type mice and n = 5 for sublytic mice, P = .0159). (C) Sections of spleens from sublytic and wild-type mice stained for iron with Prussian blue and shown at a 20× magnification. (D) Hamp expression measured by quantitative RT-PCR using total RNA isolated from liver. Expression was normalized to that of endogenous S18 transcripts (n = 5 for wild-type mice and n = 3 for sublytic mice, P = .0357). (E) Gastrointestinal iron absorption. Mice were maintained on a regular diet or on an iron-deficient diet for 1 month. Animals were fasted for 12 hours before gastric instillation of 59FeCl3. Iron absorption was determined 24 hours after gastric feeding (n = 6 for wild-type mice and n = 5 for sublytic mice). The percentage of absorption was calculated as the amount of radioactivity in the whole animal minus the gastrointestinal tract, divided by the amount instilled by gavage. Note that wild-type mice were rendered anemic, as determined by complete blood count analysis, by feeding with the iron-deficient diet for 1 month. Graphical data are expressed as means ± SEM. *P < .05.
Sublytic mice are iron deficient. (A) Serum iron levels (n = 5 for wild-type mice and n = 3 for sublytic mice, P = .0357). (B) Liver iron stores (n = 4 for wild-type mice and n = 5 for sublytic mice, P = .0159). (C) Sections of spleens from sublytic and wild-type mice stained for iron with Prussian blue and shown at a 20× magnification. (D) Hamp expression measured by quantitative RT-PCR using total RNA isolated from liver. Expression was normalized to that of endogenous S18 transcripts (n = 5 for wild-type mice and n = 3 for sublytic mice, P = .0357). (E) Gastrointestinal iron absorption. Mice were maintained on a regular diet or on an iron-deficient diet for 1 month. Animals were fasted for 12 hours before gastric instillation of 59FeCl3. Iron absorption was determined 24 hours after gastric feeding (n = 6 for wild-type mice and n = 5 for sublytic mice). The percentage of absorption was calculated as the amount of radioactivity in the whole animal minus the gastrointestinal tract, divided by the amount instilled by gavage. Note that wild-type mice were rendered anemic, as determined by complete blood count analysis, by feeding with the iron-deficient diet for 1 month. Graphical data are expressed as means ± SEM. *P < .05.
The peptide hormone hepcidin, which is encoded by Hamp, is the key regulator of iron homeostasis,17-19 responding to body iron status, inflammation, and erythropoietic activity.17,20 Hepcidin down-regulates iron absorption through the duodenal epithelium and iron release from tissue stores (particularly from macrophages in the reticuloendothelial system). We predicted that the severe iron deficiency and ongoing compensatory erythropoiesis would lead to down-regulation of hepcidin expression in sublytic mice, promoting increased iron absorption to meet the demand for iron. Quantitative RT-PCR showed that Hamp transcription was significantly down-regulated in sublytic mice, indicating that the iron-sensing pathway was intact (Figure 3D).
We then evaluated the ability of sublytic mice to absorb intestinal iron. Five-week-old male wild-type and sublytic mice were fed either regular rodent chow or an iron-deficient diet for 1 month, and then radioactive iron (59FeCl3) was introduced by intragastric feeding. Iron absorption was determined as the difference in radioactivity measured in the whole animal versus that in the gastrointestinal tract. When fed a regular diet, iron absorption was comparable between sublytic and wild-type control mice. However, whereas wild-type mice doubled their iron absorption when rendered anemic by an iron-deficient diet, homozygous sublytic mice completely failed to up-regulate iron absorption (Figure 3E). Therefore, although sublytic mice are capable of sensing iron deficiency, subsequent iron absorption is unresponsive to body iron levels. These findings strongly suggest that sublytic mice possess an intrinsic defect in iron absorption.
Anemia develops independently of the sublytic hematopoietic compartment
To determine the involvement of the hematopoietic compartment in the development of anemia, reciprocal BM transplantation was performed between wild-type and homozygous sublytic mice. Eight weeks after transplantation, blood from the reconstituted chimeric mice was collected for complete blood counts. Wild-type mice reconstituted with sublytic BM showed normal RBC indices. In contrast, sublytic homozygotes reconstituted with wild-type BM developed microcytic anemia by 8 weeks after reconstitution, as indicated by significantly decreased hemoglobin, hematocrit, and MCV value (supplemental Figure 3). In each transplantation experiment, wild-type littermates of sublytic mice were used as controls and in each case, the reconstituted mice were hematopoietically normal. These data indicate that the development of anemia is independent of the hematopoietic compartment of sublytic mice.
Interestingly, chimeric sublytic homozygotes reconstituted with wild-type BM displayed hemoglobin and hematocrit values that were slightly elevated relative to those of naive sublytic mice (supplemental Figure 3 and Table 1). A possible explanation for this is the increased RBC numbers that were observed both in Atp4asublytic/sublytic and Atp4a+/+ recipients of wild-type BM, which would result in increased hemoglobin and hematocrit values. The more severely reduced RBC parameters in nonchimeric sublytic mice may also be indicative of an intrinsic defect of sublytic erythroid cells that is conditionally manifested upon iron deficiency.
Identification of a novel Atp4a mutation in sublytic mice
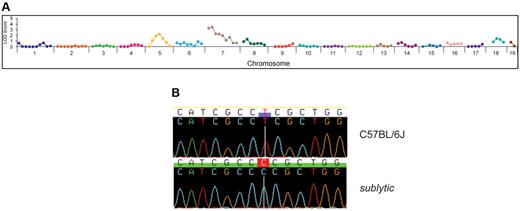
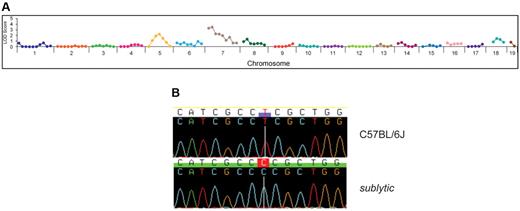
To map the sublytic phenotype, we crossed homozygous mice to wild-type C57BL/10J mice, and the resultant F1 hybrid mice were intercrossed to generate 26 F2 mice (10 displaying anemia and 16 without anemia) that were used for genetic mapping by bulk segregation analysis. Linkage was observed on chromosome 7 (Figure 4A). Genotyping of individual mice confined the critical region containing the sublytic locus to a 10-Mb interval containing 260 annotated genes.
Genetic mapping and positional cloning of sublytic. (A) Genetic linkage on chromosome 7 was established by bulk segregation analysis mapping using 26 mice (10 anemic and 16 nonanemic mice). (B) A single nucleotide transition (T863C) was detected in Atp4a. This mutation results in a serine to proline change at position 282 of the encoded gastric H+,K+-ATPase α subunit.
Genetic mapping and positional cloning of sublytic. (A) Genetic linkage on chromosome 7 was established by bulk segregation analysis mapping using 26 mice (10 anemic and 16 nonanemic mice). (B) A single nucleotide transition (T863C) was detected in Atp4a. This mutation results in a serine to proline change at position 282 of the encoded gastric H+,K+-ATPase α subunit.
Whole-genome sequencing of a sublytic homozygote using the Applied Biosystems SOLiD 3+ system produced ≥ 3× sequencing coverage of 65.4% of all coding and splice junction nucleotides. Among nucleotides covered ≥ 3×, 95 discrepancies from the reference sequence were identified, 3 of which were validated by conventional capillary sequencing (none within the critical region on chromosome 7). Within the 10-Mb critical region, 63% of coding and splice junction nucleotides were covered ≥ 3×, whereas 75% and 85.1%, respectively, were covered 2× and 1×. We sequenced conventionally 110 additional discrepancies within the critical region, which were identified among coding and splice junction nucleotides covered 1× and 2× by SOLiD sequencing. Of 80 successfully analyzed, only one point mutation was validated, a homozygous T → C transition (T863C) in Atp4a, which alters amino acid 282 of the gastric H+,K+-ATPase α subunit from serine to proline (Figure 4B). The mutation lies in the ATPase domain. Separate genotyping of 65 mice generated on the C57BL/6J background showed perfect concordance between homozygosity for the Atp4a mutation and the sublytic phenotype.
To confirm that the Atp4a mutation is responsible for the sublytic phenotype, mice heterozygous for a null allele of Atp4a were crossed with sublytic homozygotes, and RBC indices were analyzed in their offspring. At 4 weeks of age, all mice carrying one null allele and one sublytic allele of Atp4a exhibited hypochromic microcytic anemia resembling the phenotype of sublytic homozygotes (Table 2) and decreased serum iron levels (supplemental Figure 4), demonstrating that Atp4asublytic is causative for the mutant phenotype observed in sublytic mice.
Quantitative RT-PCR analysis demonstrated a 2-fold increase of Atp4a transcript in the stomach tissue of sublytic mice relative to wild-type mice (supplemental Figure 5), which is suggestive of a compensatory drive for higher gene expression.
Glandular cysts and achlorhydria develop in the stomach of sublytic homozygotes
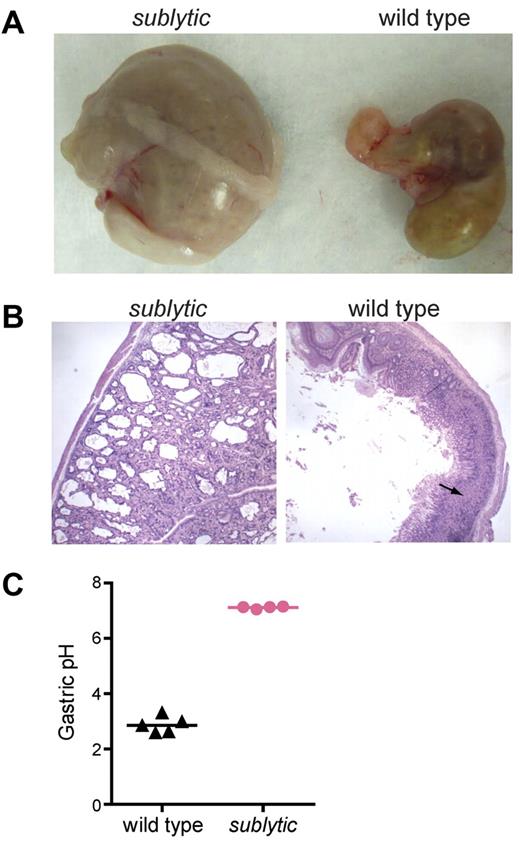
Atp4a-knockout mice were shown previously to develop achlorhydria and mucocystic and ciliated metaplasia.3,21 Similarly, the stomachs of sublytic mice were indurated and enlarged (Figure 5A). Histologic analysis revealed numerous parietal cysts in the gastric mucosa (Figure 5B), which could be observed as early as 8 weeks of age, and which increased in size and number with age. The stomach and anemia phenotypes were observed together in all homozygous sublytic mice analyzed, supporting the idea that both are caused by mutation of Atp4a.
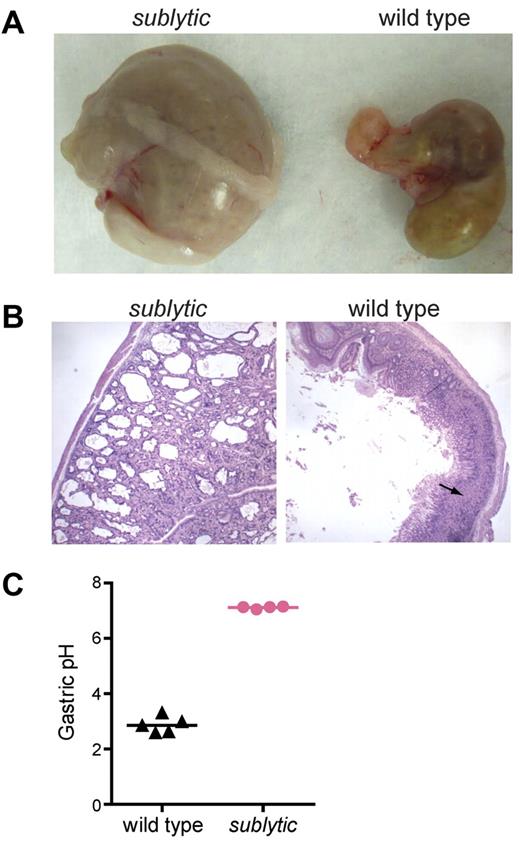
Atp4asublytic causes parietal cysts and achlorhydria. (A) Stomachs of age- and sex-matched sublytic and wild-type mice. (B) Sections of stomachs from sublytic and wild-type mice stained with H&E. Numerous parietal cell cysts developed in the gastric mucosa of sublytic mice. Arrow indicates parietal cells. (C) pH of the gastric contents of sublytic and wild-type mice. Samples were collected 45 minutes after subcutaneous injection of histamine HCl. Each point represent an individual mouse.
Atp4asublytic causes parietal cysts and achlorhydria. (A) Stomachs of age- and sex-matched sublytic and wild-type mice. (B) Sections of stomachs from sublytic and wild-type mice stained with H&E. Numerous parietal cell cysts developed in the gastric mucosa of sublytic mice. Arrow indicates parietal cells. (C) pH of the gastric contents of sublytic and wild-type mice. Samples were collected 45 minutes after subcutaneous injection of histamine HCl. Each point represent an individual mouse.
We measured the pH of the gastric contents from histamine-treated wild-type and sublytic mice. In contrast to the acidity of the gastric contents of wild-type mice, the pH of the gastric contents of sublytic mice was close to neutral (Figure 5C). This finding indicates that Atp4asublytic encodes a nonfunctional protein. Numerous reports support the importance of gastric acidity for iron solubilization and subsequent absorption by mucosal epithelial cells of the proximal small intestine.22-24 However, when sublytic mice were fed a high-iron diet for 1 month, anemia was rescued (supplemental Figure 6). The same effect was achieved by parenteral injection of iron dextran for 2 weeks (data not shown).
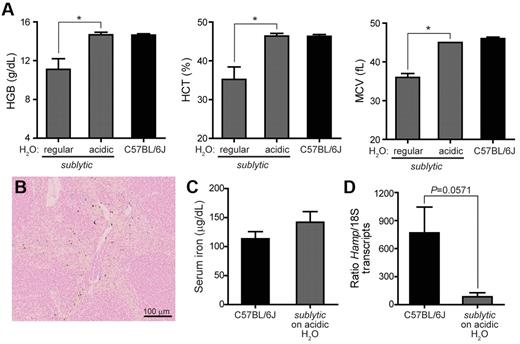
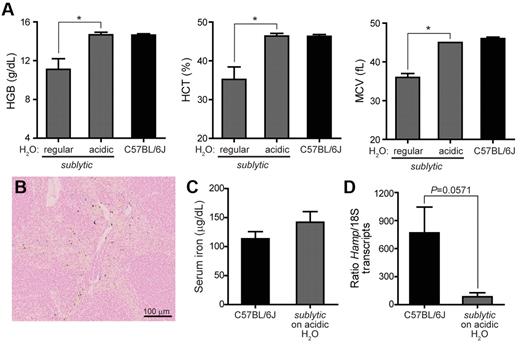
Anemia was also rescued by maintaining 2- to 4-month-old sublytic homozygous mice on HCl-acidified drinking water (pH 2.5-2.8) for 3 months. The anemia phenotype was monitored over the 3-month period. By the end of the first month, anemia was partially corrected, and RBC indices completely normalized after 3 months of treatment for all age groups (Figure 6A). Iron status also improved in sublytic mice given acidified water: iron deposits in the spleen became detectable (Figure 6B), serum iron increased to wild-type levels (Figure 6C), and hepcidin expression, although still reduced compared with wild-type levels (Figure 6D; 85 vs 768 Hamp/18S transcripts), increased 200-fold relative to that in sublytic mice maintained on nonacidified water (Figure 3D; 2 vs 3800 Hamp/18S transcripts).
Maintenance on acidified drinking water rescues anemia in sublytic mice.Sublytic homozygous mice 2-4 months of age (n = 4) were maintained for a period of 3 months on drinking water acidified to pH 2.5-2.8 with hydrochloric acid. C57BL/6J mice (n = 6) were maintained for the same period on normal drinking water. Data shown are from analysis after 3 months of treatment. (A) Complete blood counts. *P < .05 by Student t test. (B) Section of spleen from sublytic homozygote stained for iron with Prussian blue and shown at a 20× magnification. (C) Serum iron levels and (D) Hamp expression measured by quantitative RT-PCR using total RNA isolated from liver. Expression was normalized to that of endogenous 18S transcripts. P = .0571 by Student t test.
Maintenance on acidified drinking water rescues anemia in sublytic mice.Sublytic homozygous mice 2-4 months of age (n = 4) were maintained for a period of 3 months on drinking water acidified to pH 2.5-2.8 with hydrochloric acid. C57BL/6J mice (n = 6) were maintained for the same period on normal drinking water. Data shown are from analysis after 3 months of treatment. (A) Complete blood counts. *P < .05 by Student t test. (B) Section of spleen from sublytic homozygote stained for iron with Prussian blue and shown at a 20× magnification. (C) Serum iron levels and (D) Hamp expression measured by quantitative RT-PCR using total RNA isolated from liver. Expression was normalized to that of endogenous 18S transcripts. P = .0571 by Student t test.
Discussion
One-half of all cases of anemia are caused by iron deficiency,25,26 which may result from low dietary iron content, impaired iron absorption, or excessive iron loss through hemorrhage,27 among other etiologies. In the present study, we have demonstrated that a mouse mutation in the α subunit of the gastric H+,K+-ATPase causing achlorhydria leads to impaired iron absorption and consequent iron-deficiency anemia. Although homozygous sublytic mice responded to iron deficiency by down-regulating Hamp transcription, they failed to increase intestinal iron absorption, whereas wild-type animals did. We conclude that sublytic mice are capable of sensing iron deficiency, but are unable to absorb iron in response to the signals promoting its uptake. The failure in iron absorption is likely because of a combination of poor solubility of ferric iron and inefficient DMT1 function resulting from reduced availability of protons in the stomach. DMT1 transcript levels are up-regulated under conditions of iron deficiency. Consistent with this finding, we detected a nearly 3-fold increase in DMT1 transcript expression in sublytic mice (supplemental Figure 7). However, this up-regulation was not sufficient to meet iron demand and normalize iron levels.
The severity of anemia in sublytic homozygotes varied in a consistent pattern in relation to age. Anemia was most severe during the period of fast growth (4-6 weeks of age), when mice increase iron absorption to meet the demand for erythropoietic expansion. Accordingly, we observed marked reticulocytosis along with prominent iron attenuation. When body growth rate slows (after 6 weeks of age), the iron demand of erythropoiesis is reduced; moreover, the extremely low level of hepcidin in sublytic homozygotes results in high ferroportin expression on the cell membrane and therefore allows more iron to be exported from enterocytes and macrophages. As a consequence, a balance between erythropoiesis and iron availability is achieved, and milder anemia and serum iron deficiency are observed. Some of the sublytic homozygotes go on to develop a more severe form of anemia again much later in life, manifested by significantly reduced hemoglobin level and markedly elevated reticulocyte counts and splenomegaly, but the age of onset varies. Sublytic mice presumably develop chronic stomach inflammation, as has been observed in Atp4a-knockout mice,21 which may exacerbate iron deficiency and contribute to the development of severe anemia in animals older than 10 months. Currently, compound mutants generated by breeding sublytic mice to Rag1-deficient animals are being generated and may provide more insight into this phenomenon. Long-term iron deficiency may result in apoptosis of erythrocyte precursors, although this has not been observed in the mice analyzed so far. Another consequence of gastric acid suppression is the development of high levels of plasma gastrin in humans and animals,21,28,29 which may increase the risk of developing fundic gland polyps and gastric cancer.30
Surprisingly, mice deficient in the H+,K+-ATPase α subunit were not previously reported to have any hematologic abnormalities. Distinct compositions of mouse diet, housing conditions in different animal facilities, and differences in the genetic background of the Atp4a−/− knockout strain (backcrossed from a mixed 129/SvJ × Black Swiss background onto a C57BL/6J background) and sublytic mice (pure C57BL/6J background) could all potentially contribute to a difference in phenotype. However, it is not clear that the hematopoietic status of the Atp4a−/− mice was actually examined, and it is possible that they too are anemic. We demonstrate here that Atp4a mutants carrying one null allele and one sublytic allele developed anemia, strongly suggesting that the knockout allele can also cause anemia.
The long-term use of PPIs in the treatment of acid-related conditions has not been reported to result in iron deficiency in patients. In the present study, iron deficiency was observed in sublytic homozygote mice fed a diet containing only nonheme iron. Therefore, it may be that the human diet contains sufficient heme iron, which does not require an acidic environment for absorption, to supply the body's needs. In addition, ascorbic acid present in the human diet normally enhances iron absorption, but is not a common component of a regular mouse diet. Nonetheless, the use of PPIs may be considered as a potential cause of iron deficiency in certain individuals who are prone to it.9,10
In addition to iron deficiency, we found that in sublytic mice RBCs are relatively resistant to ammonium chloride lysis. The greater surface-to-volume ratio of microcytic erythrocytes may contribute to the reduced osmotic fragility observed in sublytic RBCs, which gives them more capacity to swell in hypotonic solution. Indeed, when wild-type mice were rendered iron deficient and anemic by feeding them an iron-deficient diet, their RBCs displayed smaller MCV and reduced osmotic fragility (supplemental Figure 8).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Gary E. Shull (University of Cincinnati College of Medicine, Cincinnati, OH) for kindly providing Atp4a-knockout mice; Dr Jeffrey Friedman (Department of Molecular and Experimental Medicine, The Scripps Research Institute) for helpful advice and discussions; Dr Nissi Varki (Department of Pathology, University of California-San Diego) for mouse pathology consultation; and Dr Eva Marie Y. Moresco (Department of Genetics, The Scripps Research Institute) for assistance in the preparation of the manuscript.
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to X.D. (1 R01 DK084232-01).
National Institutes of Health
Authorship
Contribution: L.K., O.M., and P.K. performed the experiments; Y.X. analyzed SOLiD whole-genome sequencing data; B.B. provided critical discussions, and X.D. devised and supervised the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for O.M. is Department of Biology, Mount St Mary's College, Los Angeles, CA.
Correspondence: Xin Du, The Scripps Research Institute, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail: xindu@scripps.edu.
References
Author notes
L.K. and O.M. contributed equally to this study.