Abstract
Clinical studies using bone marrow–derived proangiogenic cells (PACs) have demonstrated modest improvements of function and/or perfusion of ischemic myocardium or skeletal muscle. Because the identities of these PACs and their functional ability to promote neovascularization remain poorly understood, it is possible that a subset of robust PACs exists but is obscured by the heterogeneous nature of this cell population. Herein, we found that common myeloid progenitors (CMPs) and granulocyte-macrophage progenitors (GMPs) preferentially differentiate into PACs compared with megakaryocyte-erythrocyte progenitors, hematopoietic stem cells, and common lymphoid progenitors. In vivo hindlimb ischemia studies and Matrigel plug assays verified the enhanced neovascularization properties uniquely associated with PACs derived from CMPs and GMPs. Taken together, these observations identify CMPs and GMPs as key bone marrow progenitors for optimal PAC function in vitro and in vivo and provide a foundation for novel therapeutic approaches to modulate angiogenesis.
Introduction
Therapeutic angiogenesis has emerged as an innovative strategy for the treatment of patients with cardiovascular ischemic disease states, such as myocardial ischemia or peripheral artery disease. Several clinical trials using autologous, bone marrow (BM)–derived mononuclear cells have demonstrated only modest improvements of function and/or perfusion of ischemic myocardium or limb in patients.1-6 However, the identities of these BM-derived mononuclear cells and their functional ability to promote neovascularization remain poorly understood.
Accumulating evidence has indicated that proangiogenic cells (PACs) may originate from the BM and are capable of being recruited to sites of ischemic injury, where they contribute to neovascularization and tissue repair through paracrine/autocrine mechanisms or by direct incorporation into the vessel wall.7-10 Indeed, PAC function and/or number have been found to be markedly impaired in patients with coronary or peripheral artery disease.11-17 Herein, we provide evidence that a hierarchy exists among mouse hematopoietic stem cell (HSC) progenitors in their ability to differentiate into PACs and promote neovascularization. Complementary functional studies revealed that common myeloid progenitors (CMPs) and granulocyte-macrophage progenitors (GMPs) preferentially differentiate into PACs as opposed to megakaryocyte-erythrocyte progenitors (MEPs), HSCs, and common lymphoid progenitors. Moreover, only PACs derived from BM-derived CMPs and GMPs were able to enhance neovascularization in vivo. Taken together, these findings reveal a novel and unexpected hierarchy among BM-derived progenitors in their ability to promote neovascularization.
Methods
Isolation and in vitro culture of PACs
Progenitor cells were isolated from C57BL/6 mice (n = 8-10) BM by using multicolor FACSAria (BD Biosciences) as previously described.18,19 Briefly, approximately 2 to 5 × 105 BM-derived cells were prospectively isolated and purified as follows: HSCs (Lin−Sca1+c-kit+), CMPs (Lin−Sca1−c-kit+CD34+FcγRII/IIlo), GMPs (Lin−Sca1−c-kit+CD34+FcγRII/IIIhi), and MEPs (Lin−Sca1−c-kit+CD34−FcγRII/IIIlo; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Subsequently, cells were incubated in endothelial growth media-2 (EGM-2; Lonza) for 8 to 10 days and subjected to flow cytometry to detect expression of vascular endothelial growth factor receptor-2 (VEGFR2), CD31, CD133, CD34, CD45, Tie2, von Willebrand factor, endothelial nitric oxide synthase, and VE-cadherin (eBioscience). Cells were also incubated in the presence of 10 μg/mL for 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine perchlorate–acetylated low density lipoprotein (DiI-AcLDL; Biomedical Technologies) or FITC-labeled Ulex lectin (Vector Laboratories).
Endothelial network formation
Matrigel was added to 96-well culture plates, and EGM-2–cultured progenitors were admixed with human umbilical vein endothelial cells to each well at a 1:1 ratio. Six hours later, tube-like network formation was assessed.
In vivo Matrigel plug assay
Matrigel plugs admixed with 0.8 mL of Matrigel, basic fibroblast growth factor (250 ng/mL; R&D Systems), and heparin (60 units/mL), and BM-derived progenitors grown in either EGM-2 or IMDM for 7 days were implanted subcutaneously into 10-week-old C57BL/6 mice. Eight days later, explanted plugs were assessed for angiogenesis by probing paraffin sections with CD31 antibodies.
Hindlimb ischemia
Ischemic injury was produced by unilateral right femoral artery ligation in C57BL/6 mice. The deep femoral artery was cauterized, and the complete severance of the main femoral artery was performed. Immediately after surgery, mice were imaged on a Laser Doppler Imager-2. PACs were also injected intramuscularly into the right quadriceps and extent of blood flow recovery evaluated over a 14 day period.
Statistical analysis
Values are expressed as mean ± SD. Differences between values were examined using 2-tailed Student t test and were considered significant at P < .05.
Results and discussion
Identification of BM progenitor subsets that differentiate into PACs
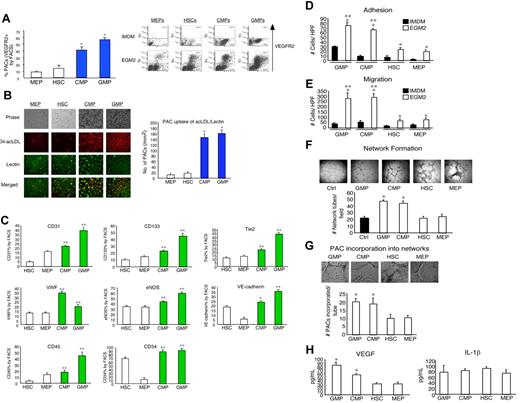
Given the fact that BM-derived mononuclear cells can promote neovascularization,4,20,21 we hypothesized that specific subsets within this population may possess distinctly high PAC differentiation potential. To assess the PAC differentiation potentials among hematopoietic stem cells or progenitors, isolated HSCs, CMPs, GMPs, MEPs, and common lymphoid progenitors were exposed to EGM-2 medium for 8 to 10 days and evaluated by flow cytometry to determine induced expression of VEGFR2 as a hallmark PAC marker. As shown in Figure 1A, CMPs and GMPs expressed approximately 4- and 6-fold higher VEGFR2 levels, respectively, than MEPs. In contrast, common lymphoid progenitors did not survive (data not shown). A similar pattern was observed among human BM-derived progenitors and human umbilical cord-derived progenitors (supplemental Figure 1B). One of the characteristics of PACs is their ability to uptake AcLDL and Ulex lectin.4,20,21 As shown in Figure 1B, PACs derived from CMPs and GMPs exhibited markedly higher uptake of DiI-AcLDL and Ulex lectin than the MEP- or HSC-derived PACs. Compared with MEP- and HSC-derived PACs, CMP- and GMP-derived PACs also expressed higher levels of the markers CD31, CD133, CD34, CD45, Tie2, von Willebrand factor, endothelial nitric oxide synthase, and VE-cadherin (Figure 1C; supplemental Figure 1C) and the chemokine receptors CXCR4, CXCR3, CCR2, and CCR7 (supplemental Figure 2A) by flow cytometry. Finally, cell growth curves revealed that CMP- and GMP-derived PACs grew up to 2-fold faster than MEP- and HSC-derived PACs over 7 days (supplemental Figure 2B-C). Collectively, these observations indicate that CMPs and GMPs have greater potential to differentiate into PACs.
Existence of a hierarchy among BM progenitors in their ability to differentiate and function as PACs. (A) BM-derived progenitors were isolated and grown in EGM-2 or IMDM medium for 7 days. VEGFR2 (KDR) expression was most induced in CMP- and GMP-derived PACs by flow cytometry. *P < .01 versus MEP. (B) CMP- and GMP-derived PACs exhibited more efficient uptake of DiI-AcLDL and Ulex lectin than MEPs and HSCs. *P < .01 versus MEPs. Images were visualized using a Nikon eclipse TE-2000-u microscope at original magnification ×10 and Spot Version 5.0 software (Diagnostics Instruments Inc). (C) PACs derived from CMPs and GMPs expressed higher levels of PAC markers CD31, CD133, CD34, CD45, Tie2, von Willebrand factor, endothelial nitric oxide synthase, and VE-cadherin by flow cytometry. *P < .05 versus HSCs. **P < .01 versus HSCs. (D-E) PACs derived from CMPs and GMPs more readily adhered to fibronectin-coated plates (D) and migrated faster than the MEPs and HSCs or the progenitors grown in IMDM (E). *P < .01 versus IMDM. **P < .01 versus MEPs. For adhesion assays, PACs (CMPs, GMPs, MEPs, and HSCs grown in EGM-2 for 7 days) were plated onto fibronectin-coated 24-well plates at a density of 20 000 cells per well. After 15 minutes of incubation at 37°C, unattached cells were removed by washing with phosphate-buffered saline, and the number of attached cells was quantitated. Migration assay was performed using a transwell system. (F) PACs derived from CMPs and GMPs promote network formation, as evidenced by Matrigel studies. The indicated PACs were incubated with human umbilical vein endothelial cells, and the number of “tubes” per high powered field was quantitated. *P < .01 versus MEPs. Images were visualized using a Nikon eclipse 80i microscope at original magnification ×4 and Spot Version 5.0 software (Diagnostics Instruments Inc). (G) PACs derived from CMPs and GMPs incorporated more efficiently into networks compared with MEPs and HSCs. *P < .01 versus MEPs. Images were visualized using a Nikon eclipse TE-2000-u microscope at original magnification ×10 and Spot Version 5.0 software (Diagnostics Instruments Inc). (H) PACs derived from CMPs and GMPs exhibited higher levels of VEGF by ELISA (left). *P < .01 versus MEPs. IL-1β (right) was not significantly different among the groups.
Existence of a hierarchy among BM progenitors in their ability to differentiate and function as PACs. (A) BM-derived progenitors were isolated and grown in EGM-2 or IMDM medium for 7 days. VEGFR2 (KDR) expression was most induced in CMP- and GMP-derived PACs by flow cytometry. *P < .01 versus MEP. (B) CMP- and GMP-derived PACs exhibited more efficient uptake of DiI-AcLDL and Ulex lectin than MEPs and HSCs. *P < .01 versus MEPs. Images were visualized using a Nikon eclipse TE-2000-u microscope at original magnification ×10 and Spot Version 5.0 software (Diagnostics Instruments Inc). (C) PACs derived from CMPs and GMPs expressed higher levels of PAC markers CD31, CD133, CD34, CD45, Tie2, von Willebrand factor, endothelial nitric oxide synthase, and VE-cadherin by flow cytometry. *P < .05 versus HSCs. **P < .01 versus HSCs. (D-E) PACs derived from CMPs and GMPs more readily adhered to fibronectin-coated plates (D) and migrated faster than the MEPs and HSCs or the progenitors grown in IMDM (E). *P < .01 versus IMDM. **P < .01 versus MEPs. For adhesion assays, PACs (CMPs, GMPs, MEPs, and HSCs grown in EGM-2 for 7 days) were plated onto fibronectin-coated 24-well plates at a density of 20 000 cells per well. After 15 minutes of incubation at 37°C, unattached cells were removed by washing with phosphate-buffered saline, and the number of attached cells was quantitated. Migration assay was performed using a transwell system. (F) PACs derived from CMPs and GMPs promote network formation, as evidenced by Matrigel studies. The indicated PACs were incubated with human umbilical vein endothelial cells, and the number of “tubes” per high powered field was quantitated. *P < .01 versus MEPs. Images were visualized using a Nikon eclipse 80i microscope at original magnification ×4 and Spot Version 5.0 software (Diagnostics Instruments Inc). (G) PACs derived from CMPs and GMPs incorporated more efficiently into networks compared with MEPs and HSCs. *P < .01 versus MEPs. Images were visualized using a Nikon eclipse TE-2000-u microscope at original magnification ×10 and Spot Version 5.0 software (Diagnostics Instruments Inc). (H) PACs derived from CMPs and GMPs exhibited higher levels of VEGF by ELISA (left). *P < .01 versus MEPs. IL-1β (right) was not significantly different among the groups.
PACs facilitate angiogenesis, in part, by their abilities to migrate, adhere, and incorporate into vascular networks, where they promote neovascularization by secreting angiogenic cytokines.4,20,21 As shown in Figure 1D-E, CMP- and GMP-derived PACs adhered to fibronectin-coated plates more efficiently and migrated faster than the MEP- or HSC-derived PACs or the CMPs and GMPs that had been grown in hematopoietic growth medium. Using the Matrigel network formation assay in vitro, BM-derived PACs were added to human umbilical vein endothelial cells; CMP- and GMP-derived PACs produced approximately 2-fold more network projections per microscopic field and incorporated into network structures at approximately 2-fold higher rate than the MEP- or HSC-derived PACs (Figure 1F-G). Finally, CMP- and GMP-derived PACs elaborated higher levels of the angiogenic growth factor VEGF compared with MEP- or HSC-derived PACs, whereas there were no differences detected in IL-1β levels (Figure 1H). Collectively, these in vitro data indicate that CMP- and GMP-derived PACs possess more favorable angiogenic properties than the MEP- and HSC-derived PACs, and even outperform CMPs and GMPs grown in hematopoietic medium alone.
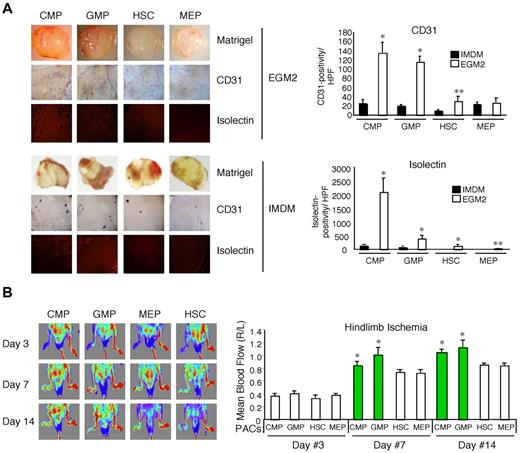
One of the central functional properties of both BM-derived mononuclear cells and PACs is their ability to promote neovascularization within complex living systems. To assess this in mice, we performed subcutaneous implantation of Matrigel with BM-derived PAC subsets grown in EGM-2 or hematopoietic progenitors grown in IMDM.22 Explanted plugs containing CMP- and GMP-derived PACs displayed up to approximately 6-fold higher CD31 staining and up to approximately 17-fold higher isolectin staining compared with plugs with MEP- and HSC-derived PACs (Figure 2A). Importantly, CMPs and GMPs grown in IMDM only minimally promoted neovascularization. To verify these findings in a pathophysiologic model of ischemic injury, PACs derived from CMPs, GMPs, MEPs, or HSCs were injected intramuscularly, immediately after femoral artery ligation in mice. As shown in Figure 2B, CMP- and GMP-derived PACs increased blood flow recovery to a greater extent than did the MEP- or HSC-derived PACs. Taken together, these findings indicate that the markedly enhanced angiogenic properties of CMP- and GMP-derived PACs are maintained in the in vivo setting.
PACs derived from CMPs and GMPs possess robust angiogenic activity in vivo. (A) Matrigel plugs admixed with BM-derived CMPs, GMPs, HSCs, or MEPs grown in either EGM-2 or IMDM were subcutaneously implanted in 10-week-old, male C57BL/6 mice (n = 4/group). Matrigel plugs were harvested 8 days later, and CD31 and isolectin staining was quantitated (right). CMPs and GMPs grown in EGM-2 possessed high angiogenic potential compared with HSCs and MEPs and progenitors grown in IMDM. *P < .01 versus IMDM. **P < .05 versus IMDM. For Matrigel plugs, Matrigel images were photographed with an Olympus, Model SZ61 camera (top). For CD31 staining (middle), images were analyzed with an Olympus, Fluoview, Model FV1000 camera at 10× magnification and FV10-ASW Version 02.01 software. For isolectin (bottom), images were analyzed using an AQUA/PM2000 Imaging Platform (HistoRx), and automated quantitative analysis was performed with Software Suite Version 2.2 (HistoRx). (B) C57BL/6 mice underwent femoral artery ligation, and PACs dervied from CMPs, GMPs, HSCs, or MEPs were immediately injected intramuscularly at the ligation site. Blood flow recovery as visualized by laser Doppler imaging (785-nm near-infrared laser Doppler Imager-2; Moor Instruments) demonstrated increased ratios (ratio of ischemic thigh/contralateral thigh) in mice injected with PACs from CMPs and GMPs at days 7 and 14. *P < .05 versus MEP.
PACs derived from CMPs and GMPs possess robust angiogenic activity in vivo. (A) Matrigel plugs admixed with BM-derived CMPs, GMPs, HSCs, or MEPs grown in either EGM-2 or IMDM were subcutaneously implanted in 10-week-old, male C57BL/6 mice (n = 4/group). Matrigel plugs were harvested 8 days later, and CD31 and isolectin staining was quantitated (right). CMPs and GMPs grown in EGM-2 possessed high angiogenic potential compared with HSCs and MEPs and progenitors grown in IMDM. *P < .01 versus IMDM. **P < .05 versus IMDM. For Matrigel plugs, Matrigel images were photographed with an Olympus, Model SZ61 camera (top). For CD31 staining (middle), images were analyzed with an Olympus, Fluoview, Model FV1000 camera at 10× magnification and FV10-ASW Version 02.01 software. For isolectin (bottom), images were analyzed using an AQUA/PM2000 Imaging Platform (HistoRx), and automated quantitative analysis was performed with Software Suite Version 2.2 (HistoRx). (B) C57BL/6 mice underwent femoral artery ligation, and PACs dervied from CMPs, GMPs, HSCs, or MEPs were immediately injected intramuscularly at the ligation site. Blood flow recovery as visualized by laser Doppler imaging (785-nm near-infrared laser Doppler Imager-2; Moor Instruments) demonstrated increased ratios (ratio of ischemic thigh/contralateral thigh) in mice injected with PACs from CMPs and GMPs at days 7 and 14. *P < .05 versus MEP.
Clinical trials applying various PAC subsets for therapeutic angiogenesis of myocardial or peripheral artery disease have thus far achieved modest functional effects,1 or in some cases, the absence of any dose-response effect.5,6 Because heterogeneity of the PACs used in this therapeutic strategy may be obscuring the identities and function of more potent subsets, identifying those cells with significantly robust angiogenic activity is necessary to the efficacy of PAC-based protocols in treating ischemic cardiovascular disease. This study demonstrated that CMP- and GMP-derived PACs possess these robust angiogenic properties and may provide an opportunity to improve cell-based therapies for ischemic cardiovascular disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (HL080174 and HL091076, M.W.F.; and F32HL088819, A.K.W.), the American Cancer Society (Research Scholar Grant RSG0719501-LIB, M.W.F.), the Boston Area Diabetes Endocrinology Research Center (Pilot Grant P30DK057521, M.W.F.), and a Watkins Cardiovascular Medicine Discovery Award (M.W.F.). A.R. is a principal faculty member of the Harvard Stem Cell Institute. S.Y.F. was supported by the Clinical Investigators Training Program (Beth Israel Deaconess Medical Center–Harvard/MIT Health Sciences and Technology) in collaboration with Pfizer Inc and Merck & Co. This work was also supported in part by the Leducq Foundation Network of Research Excellence (A.R. and S.Y.F).
National Institutes of Health
Authorship
Contribution: A.K.W. designed experiments, performed research, analyzed and interpreted data, and wrote the manuscript; K.C., S.Y.F., X.S., B.I., and F.E. performed research and analyzed the data; Y.T. performed research; A.R. interpreted the data and edited the manuscript; and M.W.F. designed experiments, performed research, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: M.W.F. and the Brigham and Women's Hospital have a patent pending related to the work that is described in the present study. The remaining authors declare no competing financial interests.
Correspondence: Mark W. Feinberg, Department of Medicine, Cardiovascular Division, Brigham and Women's Hospital, Harvard Medical School, 77 Ave Louis Pasteur, NRB-742F, Boston, MA 02115; e-mail: mfeinberg@rics.bwh.harvard.edu.