In this issue of Blood, Yau and colleagues provide evidence that guide catheter thrombosis in patients undergoing percutaneous coronary intervention (PCI) is initiated by contact system activation, and that unfractionated heparin is more effective than fondaparinux at limiting catheter occlusions because heparin-antithrombin complexes are able to target multiple points along the coagulation cascade, as opposed to the smaller fractionated heparins and heparinoids that provide higher selectivity for factor Xa.1
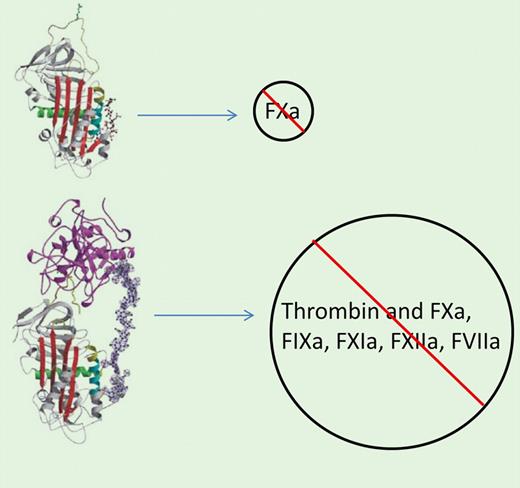
Catheter thrombosis during PCI procedures is a significant threat that has been reasonably addressed using interventional doses of unfractionated heparin. The recent shift in clinical practice toward the use of low molecular weight heparins (LMWH) and heparinoids for systemic anticoagulation, including the small synthetic pentasaccharide molecule fondaparinux, has surprisingly not translated to reliable inhibition of catheter thrombosis. Recent data indicate that fondaparinux is less effective than heparin in preventing guide catheter thrombosis,2 which poses a mechanistic puzzle. Yau et al show experimental data that provide a reasonable explanation for why heparin, at clinically relevant antithrombotic concentrations, is more effective at preventing catheter-initiated thrombosis than fondaparinux. On complex formation with antithrombin and several other serpins, linear sulfated glycosaminoglycan molecules accelerate the inhibition of numerous serine proteases,3 including those of the coagulation cascade, ranging from factors VIIa and XIa to thrombin.4 Unfractionated heparin is a mixture of sulfated glycosaminoglycans of different sizes that variably alter the structure and charge density of antithrombin molecules, making them accessible as suicide substrates to select serine proteases. While larger heparin fragments complexed with antithrombin are excellent thrombin inhibitors, smaller heparin fragments cannot effectively bridge antithrombin to thrombin,5 though they are potent factor Xa (FXa) inhibitors (see figure). The present study demonstrates that PCI catheter materials can promote thrombus formation through activation of the contact system and the intrinsic pathway when they come in contact with blood. Catheter-induced contact activation results in robust generation of FXa in plasma, and this flood of FXa is apparently able to bypass fondaparinux, and to a lesser extent enoxaparin, at otherwise antithrombotic concentrations. Indeed, FXa, once assembled into the prothrombinase complex, is protected from antithrombin-fondaparinux and antithrombin-enoxaparin.6 Unfractionated heparin, acting like multiple dams on a flooding river, can block both the direct actions of thrombin as well as thrombin generation, and thus more effectively modulates the thrombogenic challenge of PCI procedures. Yau et al's study suggests that in interventional cardiology, LMWH and small heparinoids like fondaparinux still have reason for “heparin envy,” and need additional help from heparin, direct thrombin inhibitors, or other antithrombotic agents to complete the job.
(A) Binding of the pentasaccharide fondaparinux to antithrombin induces a conformational change, increasing the inactivation primarily of FXa. (B) Complex between antithrombin and heparin greatly enhances the inactivation of thrombin as well as several additional activated coagulation factors. From Li et al.5
(A) Binding of the pentasaccharide fondaparinux to antithrombin induces a conformational change, increasing the inactivation primarily of FXa. (B) Complex between antithrombin and heparin greatly enhances the inactivation of thrombin as well as several additional activated coagulation factors. From Li et al.5
Current antithrombotics, including heparin and thrombin inhibitors, target essential hemostatic factors and therefore predictably increase bleeding risks. The apparent improved overall safety of novel antithrombotic agents may sometimes sacrifice antithrombotic efficacy. A future alternative may bring about the development and use of truly biocompatible devices that do not trigger contact activation-dependent pathologic events. Until then, establishing and carefully balancing the efficacy and safety of drug combinations, as suggested by this study, may be our best option during PCI.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■