Abstract
Primary CNS lymphoma (PCNSL) is a rare malignancy with peculiar clinical and biologic features, aggressive course, and unsatisfactory outcome. It represents a challenge for multidisciplinary clinicians and scientists as therapeutic progress is inhibited by several issues. Molecular and biologic knowledge is incomplete, limiting the identification of new therapeutic targets, and the particular microenvironment of this malignancy, and sanctuary sites where tumor cells grow undisturbed, strongly affects treatment efficacy. Moreover, active treatments are known to be associated with disabling neurotoxicity, posing the dilemma of whether to intensify therapy to improve the cure rate or to de-escalate treatment to avoid sequels. The execution of prospective trials is also difficult because of the rarity of the tumor and the impaired general condition and poor performance status of patients. Thus, level of evidence is low, with consequent uncertainties in therapeutic decisions and lack of consensus on primary endpoints for future trials. Despite this unfavorable background, laboratory and clinical researchers are coordinating efforts to develop new ideas, resulting in the recent publication of studies on PCNSL's biology and molecular mechanisms and of the first international randomized trials. Herein, these important contributions are analyzed to provide recommendations for everyday practice and the rationale for future trials.
Introduction
Primary CNS lymphoma (PCNSL) is an aggressive malignancy arising exclusively in the CNS, that is, the brain parenchyma, spinal cord, eyes, cranial nerves, and/or meninges.1 This lymphoma represents 4% of intracranial neoplasms and 4%-6% of all extranodal lymphomas but some registry studies suggest that its incidence in immunocompetent patients is progressively increasing.2 PCNSL exhibits peculiar clinical and biologic features and constitutes a diagnostic and therapeutic challenge for multidisciplinary clinicians and scientists. Its outcome remains unsatisfactory if compared with that of patients with extra-CNS lymphomas of a similar stage and histotype, and several factors prevent therapeutic progress. First, molecular and biologic knowledge is insignificant compared with other lymphomas, which limits the identification of new therapeutic targets. Second, patients with PCNSL usually exhibit impaired general condition and poor performance status (PS) more often than subjects with other lymphomas.1 This usually interferes with the enrollment of patients in prospective trials. In fact, current therapeutic knowledge is only based on 3 randomized trials, some single-arm phase 2 trials, anecdotal meta-analyses, and a few multicenter retrospective studies. Thus, available level of evidence is low, with consequent uncertainties in therapeutic decisions and lack of consensus on primary endpoints for future trials.
In this manuscript, I discuss available clinical and biologic data on PCNSL in immunocompetent patients and provide recommendations for everyday practice and the rationale for future trials.
Clinical presentation and imaging
Which are the most common presentations?
In immunocompetent patients,3,4 the median age at diagnosis is 60 years, with a male:female ratio of 1.2:1.7. The most common symptoms, which often span weeks to months, are focal deficits, personality changes, and increased intracranial pressure (Figure 1). B symptoms are rare.3 Intraocular involvement is observed in 10%-20% of patients. It can be the sole expression of lymphoma (primary intraocular lymphoma), or can be followed by the onset of brain lesions after weeks or months. Tumor cells can infiltrate the vitreous humor, the retina, the choroid, and optic nerve. Only half of these patients experience visual complaints such as floaters, blurred vision, visual field defects, and diminished visual acuity.
Flow chart of management of PCNSL from presentation to therapeutic decision in ordinary clinical practice. MRI indicates magnetic resonance imaging; CT, computerized tomography; CSF, cerebrospinal fluid; LDH, lactate dehydrogenase serum level; and ADC, average diffusion coefficient. Deep regions refers to basal ganglia, corpus callosum, periventricular areas, brain stem, and/or cerebellum. (1) Ocular examination should include slit-lamp examination, indirect ophthalmoscopy,and ophthalmic ultrasonography. (2) Cerebrospinal fluid evaluation should include cell counts, protein and glucose levels, cytology, flow cytometry, and IgHV gene rearrangement studies.
Flow chart of management of PCNSL from presentation to therapeutic decision in ordinary clinical practice. MRI indicates magnetic resonance imaging; CT, computerized tomography; CSF, cerebrospinal fluid; LDH, lactate dehydrogenase serum level; and ADC, average diffusion coefficient. Deep regions refers to basal ganglia, corpus callosum, periventricular areas, brain stem, and/or cerebellum. (1) Ocular examination should include slit-lamp examination, indirect ophthalmoscopy,and ophthalmic ultrasonography. (2) Cerebrospinal fluid evaluation should include cell counts, protein and glucose levels, cytology, flow cytometry, and IgHV gene rearrangement studies.
PCNSL tends to infiltrate the subependymal tissues, disseminating through the cerebrospinal fluid to the meninges. Concurrent meningeal involvement, often asymptomatic, is detected by conventional cerebrospinal fluid cytology examination in 16% of patients,3 while isolated leptomeningeal lymphoma represents < 5% of all PCNSL. Spinal cord lymphoma is the rarest manifestation of PCNSL.3 This lymphoma often arises in the upper thoracic or lower cervical regions of the spinal cord and presenting symptoms depend on the level of the spinal cord involved. Their prognosis is poor mainly because of delayed diagnosis and the sparse literature suggests that they should be treated similarly to other PCNSL. Lymphomas arising in the spinal nerves and ganglia (“neurolymphomatosis”), cauda equina, and the sciatic nerve are extremely rare.5 Diagnosis is confirmed by nerve biopsy in 88% of cases, with positive cerebrospinal fluid cytology in 40% of patients. Neurolymphomatosis should be distinguished from neural infiltration by a systemic lymphoma.
Which is the best neuroimaging modality?
The above-mentioned symptoms usually lead to neuroimaging assessment. Contrast-enhanced cranial magnetic resonance imaging (MRI) is the best imaging modality for assessing PCNSL patients. Lesions are often isointense to hypointense on T2-weighted MRI, with variable surrounding edema and a homogeneous and strong pattern of enhancement (Figure 1–2).6 In cases of MRI contraindications, contrast-enhanced cranial computed tomography (CT) scans are recommended. PCNSL presents as a solitary intracranial mass lesion in 60%-70% of patients, mostly located in the hemispheres, basal ganglia, corpus callosum, and periventricular regions (Figure 1). Gliomas, metastases, toxoplasmosis, sarcoidosis, and progressive multifocal leukoencephalopathy are the main differential diagnoses, requiring brain biopsy for definitive diagnosis.
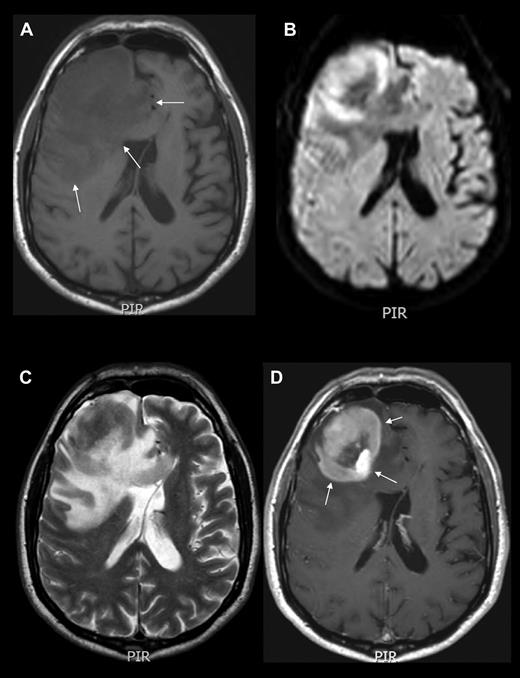
Neuroimaging example of PCNSL. MRI of the brain showing an expansive mass lesion in the right frontal lobe, which is hypointense in noncontrasted T1 scans (A), isointense with respect to cortex in T2-weighted images (C), with reduced average diffusion coefficient (B), and homogeneous contrast enhancement in contrasted T1 weighted scans (D arrows). Lesion is surrounded by modest edema (A arrows). CT and MRI findings are attributed to the high cell density and scant cytoplasm. Enhancement along the Virchow-Robin spaces, although not constant, is a highly specific feature of PCNSL.
Neuroimaging example of PCNSL. MRI of the brain showing an expansive mass lesion in the right frontal lobe, which is hypointense in noncontrasted T1 scans (A), isointense with respect to cortex in T2-weighted images (C), with reduced average diffusion coefficient (B), and homogeneous contrast enhancement in contrasted T1 weighted scans (D arrows). Lesion is surrounded by modest edema (A arrows). CT and MRI findings are attributed to the high cell density and scant cytoplasm. Enhancement along the Virchow-Robin spaces, although not constant, is a highly specific feature of PCNSL.
Diagnosis
When is tumor resection indicated?
Stereotactic-guided biopsy is the chosen method to diagnose PCNSL. Its morbidity is very low and permits the rapid detection of tumor cells in intraoperative analysis; however, samples are small and it could have a negative effect on diagnosis and biologic investigations. Patients with a radiologic suspicion of gliomas, metastasis, meningiomas, or other tumors are routinely referred for complete resection, sometimes resulting in an unexpected diagnosis of lymphoma. Conversely, I advise strongly against gross tumor resection if PCNSL is suspected because it can induce neurologic deficits and treatment delays and has not been associated with survival benefits according to a meta-analysis of 50 published prospective and retrospective series.7 In these patients, tumor resection should be reserved for the timely control of neurologic deterioration because of brain herniation or ventricle dilation to provide prompt treatment in “fit” patients.
Should steroids be interrupted before biopsy?
As with most patients who have been newly diagnosed with an intracranial mass, steroids are also routinely prescribed to PCNSL patients to rapidly improve neurologic performance and to reduce the risk of complications. Mass shrinking is obtained through antiedema and cytotoxic effects, causing radiographic regression in ∼ 40% of patients (“vanishing tumor”), which is suggestive for PCNSL. As many others, I believe that detection of a “vanishing tumor” should not be considered as diagnostic of PCNSL because sarcoidosis, multiple sclerosis, acute encephalomyelitis, and other malignancies can also exhibit a dramatic response to steroids.8
Steroid effects can interfere with histopathologic diagnosis. Although some authors reported that PCNSL can be successfully diagnosed without stopping steroids,9 there is consensus that these drugs should be withheld in patients with a presumptive diagnosis of PCNSL until tissue is obtained, limiting its use to cases of osmotherapy inefficacy.10 In our institution, we interrupt steroid therapy for at least 7-10 days before biopsy to improve diagnostic yield. However, this measure is not needed in patients who experience MRI-confirmed disease progression under steroids.
Steroid administration during antilymphoma therapy should be modified according to clinical requirements. During chemotherapy, I recommend maintaining steroids until radiologic confirmation of response, and then tapering them as soon as possible to reduce their immunosuppressive effect. I am against any superfluous use of steroids, which aims to reassure physicians rather than to control symptoms or prevent complications, even in prospective trials, where response to steroids can be erroneously attributed to investigational chemotherapy activity, generating important interpretation biases.
Which are the most important histopathologic and molecular features?
Ninety-five percent of PCNSLs are diffuse large B-cell lymphomas (DLBCL). Neoplastic B lymphocytes usually grow forming perivascular cuffings (Figure 3), with an almost constant expression of pan-B-cell markers and markers of germinal center and late germinal center B cells, while are rarely positive for CD10 (< 10%), and are negative for EBV.11 Proliferating index is usually high. Other lymphoma categories like Burkitt, lymphoblastic, marginal zone, and small lymphocytic lymphoma are uncommon differential diagnoses. T-cell PCNSLs are ∼ 2% of cases in Western countries3 and, in my opinion, should be treated similarly to standard PCNSL, with the obvious exclusion of anti-CD20 agents, and expecting similar results.12 Neuroepithelial tumors, metastatic lesions, histiocytic lesions, and inflammatory disorders like vasculitis and multiple sclerosis are the main nonlymphomatous differential diagnoses.
Classic histopathologic picture of PCNSL of diffuse large B-cell lymphoma category. Tumor cells (filled arrows) usually grow forming classic perivascular cuffings, are CD20 positive, and express other pan-B-cell markers (CD19, CD20, CD22, CD79a), markers of germinal center B cells (bcl-6; 60%-80% of cases), and markers of late germinal center B cells (MUM1; 90%), while are CD3 negative. A reactive T-cell infiltrate (open arrows) constituted by a perivascular rim of small lymphocytes interposed between vessel (VL) and neoplastic cells is observed in one-third of cases. These lymphocytes are smaller than neoplastic ones, and are CD20 negative and CD3 positive. The presence of this reactive infiltrate is associated with significantly better outcome in patients treated with modern approach.
Classic histopathologic picture of PCNSL of diffuse large B-cell lymphoma category. Tumor cells (filled arrows) usually grow forming classic perivascular cuffings, are CD20 positive, and express other pan-B-cell markers (CD19, CD20, CD22, CD79a), markers of germinal center B cells (bcl-6; 60%-80% of cases), and markers of late germinal center B cells (MUM1; 90%), while are CD3 negative. A reactive T-cell infiltrate (open arrows) constituted by a perivascular rim of small lymphocytes interposed between vessel (VL) and neoplastic cells is observed in one-third of cases. These lymphocytes are smaller than neoplastic ones, and are CD20 negative and CD3 positive. The presence of this reactive infiltrate is associated with significantly better outcome in patients treated with modern approach.
Molecular studies focused on PCNSL development showed some interesting features, such as a high load of somatic mutations, frequent ongoing somatic hypermutation patterns and biased usage of VH gene segments (IGHV4-34 rearranged in 50%-80% of cases), suggesting a role for antigen(s) triggering13,14 ; important differences in IG and BCL6 translocations with respect to nodal DLBCL15 ; mutations in oncogene and tumor suppressor gene loci (CD95, CMYC13, PAX5, PIM1, PRDM114, and TTF)15 ; and deregulation of specific pathways (NF-κB, CARD11, MALT1, and p50).16,17 Moreover, studies of chemokines showed that CXCL9/CXCL12 coexpression is a strong chemoattractant stimulus for both CXCR4+/CXCR3+/CD8+ tumor-infiltrating lymphocytes and CXCR4+/CXCR3− malignant B cells in the perivascular microenvironment.18 Gene expression profiling (GEP), array comparative genomic hybridization, and single-nucleotide polymorphism (SNP)–chip analyses were used to characterize biologic mechanisms. SNP and GEP results appear consistent and suggest an improved proliferation and harmed apoptosis in tumor cells; however, the comprehensive interpretation of resulting data are far from being established. GEP results on a CNS signature were conflicting, mostly because of the small size of investigated samples and differences in platforms and algorithms.19-21 Gene silencing because of epigenetic phenomena like CpG islands promoter hypermethylation could be related to lymphomagenesis and lymphocyte motility. Some of them, such as RFC and MGMT, may have important therapeutic implications.22,23
Staging and pretreatment evaluations
Which staging procedures should be performed?
By definition, PCNSL is a stage I disease. The involvement of different CNS areas such as eyes, meninges, and/or cranial nerves, does not imply a more advanced stage with a worse prognosis. Pretreatment “staging” procedures have 2 main goals in PCNSL: to define the extension of the disease in different CNS structures and to exclude concomitant systemic lymphoma. Conventional lymphoma staging demonstrates the presence of extraneural disease in 4%-12% of patients with presumptive diagnosis of PCNSL.24 Specific baseline evaluations and staging have been standardized by The International PCNSL Collaborative Group (Figure 1).10 During staging, we pay particular attention to ophthalmic examinations, which allow the detection of asymptomatic ocular involvement in 5% of PCNSLs. The suspicion of ocular infiltration should be confirmed by vitrectomy for cytologic examination and flow cytometry, mostly in patients with primary intraocular lymphoma. IgHV gene rearrangements analyses and intravitreal levels of IL-10 and IL-6 are, probably, adjuncts for intraocular lymphoma diagnosis.25 Cerebrospinal fluid sampling should be performed in every patient with suspected or confirmed PCNSL. Increases in the white blood cell count and protein concentrations are often present, while glucose concentration is usually normal.3 Cytology and PCR should be regarded as complementary methods because of the high rate of discordant results.26 A contrasted whole-spine MRI may be useful if lumbar puncture is contraindicated. I recommend that a testicular ultrasound examination is performed in elderly patients because of frequent CNS involvement in testicular lymphomas. The clinical relevance of positron emission tomography (PET) requires prospective evaluation. In a retrospective study,27 18FDG-PET disclosed concomitant systemic lymphoma in 7% of patients with a presumptive diagnosis of PCNSL, with detection of other malignancies in 5% of cases and false positives in 13%.
Are there reliable prognostic factors?
The identification of reliable prognostic factors is the first step toward a risk-tailored treatment of PCNSL. Virtually all studies confirm the importance of age and PS, whereas the International Prognostic Index does not discriminate among risk groups. The combination of 5 independent predictors of response and survival, that is, age, PS, serum lactate dehydrogenase level, cerebrospinal fluid protein concentration, and the involvement of deep structures (Figure 1), distinguishes 3 risk groups based on the presence of 0-1, 2-3, or 4-5 unfavorable features (IELSG [International Extranodal Lymphoma Study Group] prognostic score).28 Currently, we include the IELSG score as a stratification criterion in randomized trials, and I strongly recommend its use in choosing individualized risk-tailored treatment. Another scoring system that only uses age and PS also distinguishes 3 risk groups.29 Several biochemical, histopathologic, and molecular variables were suggested as prognostic indicators. However, none of these observations were confirmed.
First-line treatment
For decades, radiotherapy was the exclusive treatment for patients with PCNSL, while the addition of chemotherapy has significantly improved their outcome. Even though this was not confirmed in a randomized trial, there is consensus that combined chemoradiotherapy is superior to radiotherapy alone and this is the most commonly used approach.1 With variable chemotherapy regimens and radiation doses, upfront chemoradiotherapy has been addressed in several trials, obtaining complete remission rates (CRR) of 30%-87% and 5-year overall survival (OS) rates of 30%-50% (Table 1). These strategies are often associated with severe neurotoxicity, especially among elderly patients. Therefore, the dilemma posed by PCNSL treatment is the choice between strategies designed to intensify therapy to improve cure rate and treatment de-escalation strategies to avoid neurotoxicity.
Chemotherapy
Chemotherapy plays a central role in the management of PCNSL. Its efficacy is limited by several factors including the biology and microenvironment of this malignancy, which is protected by the blood-brain barrier (BBB). This results in the formation of chemotherapy sanctuaries, such as cerebrospinal fluid, meninges, and eyes, where tumor cells grow undisturbed. For this reason, I suggest taking into account the ability to cross the BBB and achieve therapeutic concentrations in the CNS as an important drug selection parameter for treating PCNSL patients. Most regimens include drugs able to cross the BBB at conventional doses (ie, steroids, some alkylating agents) and cytostatics with low to moderate ability to cross the BBB that can be safely administered at high doses to improve CNS bioavailability (ie, methotrexate, cytarabine). Conversely, drugs with poor BBB penetration that cannot be administered at high doses because of dose-limiting toxicity (ie, anthracyclines, vinca-alkaloids) are inefficient in PCNSL.
Does CHOP regimen play any role in PCNSL?
CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimen represents the backbone for treating extra-CNS DLBCL but exhibits negligible activity in PCNSL; this has been confirmed in a randomized trial with incomplete accrual.30 In retrospective series, the addition of CHOP to high-dose methotrexate (HD-MTX) resulted in higher toxicity without improved outcome compared with HD-MTX alone.31 Most patients treated with CHOP have an immediate radiographic response followed by early progression, probably because of the normalization of the disrupted BBB. This suggests that the bulky tumor not protected by the BBB responds, while the microscopic tumor is not adequately treated and progresses. In line with this evidence, we have abandoned CHOP chemotherapy in classic PCNSL. However, I prescribe CHOP-rituximab combined with good CNS bioavailability agents to patients with neurolymphomatosis5 or intravascular large B-cell lymphoma with CNS involvement32 as tumor cells of these lymphomas mostly grow in structures (nerves and blood vessels, respectively) variably, or not, protected by physiologic barriers (Figure 4).
Flow chart of therapeutic management of PCNSL in everyday practice. (1) Mostly marginal zone B-cell lymphoma, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma. (2) Mostly intravascular large B-cell lymphoma and neurolymphomatosis. (3) Conclusion from the IELSG no. 20 trial.40 (4) Several regimens are available (Table 3). (5) A higher amount of available evidence suggests WBRT. The discussion with selected patients about the pros and cons of the use of consolidation WBRT or HDC/ASCT is recommended. (6) Available literature suggesting that some elderly patients in CR after primary chemotherapy could be watchful waited without OS impairment is constituted by a few small retrospective series. However, to delay WBRT until relapse is an acceptable strategy considering the increased risk of disabling neurotoxicity in these patients. (7) Radiation field and dose should be chosen on the bases of response to primary chemotherapy. WBRT dose reduction to 23-30 Gy in patients in CR after chemotherapy is recommended. DLBCL indicates diffuse large B-cell lymphoma; HD-MTX, high-dose methotrexate; ara-C, cytarabine; WBRT, whole-brain radiotherapy; CR, complete remission; PR, partial response; SD, stable disease; PD, progressive disease; and HDC/ASCT, high-dose chemotherapy supported by autologous stem cell transplantation.
Flow chart of therapeutic management of PCNSL in everyday practice. (1) Mostly marginal zone B-cell lymphoma, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma. (2) Mostly intravascular large B-cell lymphoma and neurolymphomatosis. (3) Conclusion from the IELSG no. 20 trial.40 (4) Several regimens are available (Table 3). (5) A higher amount of available evidence suggests WBRT. The discussion with selected patients about the pros and cons of the use of consolidation WBRT or HDC/ASCT is recommended. (6) Available literature suggesting that some elderly patients in CR after primary chemotherapy could be watchful waited without OS impairment is constituted by a few small retrospective series. However, to delay WBRT until relapse is an acceptable strategy considering the increased risk of disabling neurotoxicity in these patients. (7) Radiation field and dose should be chosen on the bases of response to primary chemotherapy. WBRT dose reduction to 23-30 Gy in patients in CR after chemotherapy is recommended. DLBCL indicates diffuse large B-cell lymphoma; HD-MTX, high-dose methotrexate; ara-C, cytarabine; WBRT, whole-brain radiotherapy; CR, complete remission; PR, partial response; SD, stable disease; PD, progressive disease; and HDC/ASCT, high-dose chemotherapy supported by autologous stem cell transplantation.
Which is the backbone of upfront chemotherapy?
Antimetabolites such as MTX and cytarabine (ara-C) constitute the backbone of most anti-PCNSL regimens with proven efficacy in prospective trials (Table 1). MTX doses up to 8 g/m2 are feasible in PCNSL patients; both dose and infusion rate are important for MTX delivery to the brain parenchyma and cerebrospinal fluid.33 MTX doses ≥ 1 g/m2 result in tumoricidal levels in the brain parenchyma and doses ≥ 3 g/m2 yield tumoricidal levels in the cerebrospinal fluid.34 Conversely, doses up to 8 g/m2 delivered in a 24-hour continuous infusion do not achieve an adequate cerebrospinal fluid level.35 HD-MTX (8 g/m2) monochemotherapy yielded activity and efficacy similar to those recorded in trials assessing polychemotherapy with a MTX dose of 3.5 g/m2, but it requires more frequent dose reductions because of impaired creatinine clearance.36 As many others, I believe tolerability and activity of MTX 3.5 g/m2 suggest this may be a good compromise between safety and efficacy for combination regimens. In the future, individualized dosing of MTX using creatinine clearance or glomerular filtration rates may have the potential to improve outcome in PCNSL patients.33,37,38
Noncomparative studies suggesting an improvement of survival by adding HD–ara-C to HD-MTX3,39 were the background of the first randomized trial with completed accrual in PCNSL.40 In this trial, named IELSG no. 20, 79 patients have been assigned to 4 courses of MTX 3.5 g/m2, alone or combined with ara-C (4 doses of 2 g/m2) in both arms followed by whole-brain irradiation (WBRT). The addition of ara-C has resulted in significantly improved response (CRR = 46% vs 18%; P = .006) and survival rates (3-year OS: 46% vs 32%; P = .07) compared with HD-MTX alone (Table 1), with manageable hematologic toxicity and uncommon nonhematologic side effects.40 A recent study highlighted the importance of ara-C dose, suggesting that 4 doses of 2 g/m2 is an appropriate choice.41 Although it was not addressed in a phase 3 trial, the MTX-ara-C combination is the current standard chemotherapeutic approach for de novo PCNSL as it is supported by the highest level of evidence available (Figure 4).
Are there other active drugs?
Some active drugs have emerged from prospective studies and are being tested in ongoing trials of new first-line combinations. Temozolomide, an oral alkylating agent largely used in neuro-oncology, is the best example. It has been associated with excellent tolerability, 31% CRR and 1-year OS of 31% in patients with PCNSL relapsed or refractory to HD-MTX.42 As upfront monotherapy, temozolomide was associated with 47% CRR, and median OS of 21 months in elderly patients.23 The combination of temozolomide with HD-MTX was associated with encouraging results, even among elderly patients.43,44 Topotecan, a topoisomerase-I inhibitor, is another example. It is active in relapsed PCNSL. However, I do not prescribe this drug as its responses are short-lived (1-year PFS: 13%) and it is frequently associated with severe leukopenia and neurologic deterioration.45
Is there a role for rituximab in the treatment of PCNSL?
Rituximab is used in PCNSL patients because of its positive effect in extra-CNS DLBCL patients. However, as a large protein, it shows poor CNS penetration and its ability to prevent CNS dissemination of DLBCL remains debatable. Promising effects of rituximab were reported, both as salvage monotherapy46 (Table 4) and combined with HD-MTX47 (Table 1). Although the level of evidence supporting rituximab for the treatment of PCNSL remains very low, its use is encouraged by several hematologists. I believe rituximab should only be used in PCNSL patients in prospective trials, at least until its role is defined by the 2 large ongoing randomized trials focused on this issue (NCT01011920; NTR2427).
Is intrathecal chemotherapy needed?
Cerebrospinal fluid acts as a reservoir for PCNSL tumor cells; the persistence of tumor cells in this chemotherapy sanctuary results in increased risk of failure. Some authors advised including intrathecal/intraventricular drug delivery as part of primary chemotherapy to improve disease control. However, this suggestion is questionable as systemic HD-MTX can clear the cerebrospinal fluid of neoplastic cells. Moreover, intrathecal/intraventricular chemotherapy has not been studied prospectively, only one phase 1 trial suggests transient activity of intraventricular rituximab.50 Two large retrospective studies have not demonstrated benefits from adding intrathecal drug delivery in patients treated with HD-MTX.3,51 Conversely, the comparison of 2 single-arm trials seems to suggest some benefit from intraventricular chemotherapy.52,53 In the first trial,52 65 PCNSL patients were treated with MTX–ara-C–based chemotherapy combined with intraventricular delivery of MTX, ara-C, and steroids, resulting in a median EFS of 21 months, with 57% of young patients alive at a median follow-up of 100 months. In the second trial,53 18 patients received the same chemotherapy without intraventricular treatment to reduce Ommaya reservoir infections. Despite a similar response rate, more than half of the responders relapsed within the first year, prompting premature termination of the trial, which was attributed to the omission of intraventricular chemotherapy. Overall, there is no consensus on cerebrospinal fluid prophylaxis and treatment but most recently reported or ongoing PCNSL trials do not use intrathecal/intraventricular chemotherapy. In our institution, we do not use intrathecal/intraventricular chemotherapy in PCNSL patients as it is based on a low level of evidence and is associated with additional risk of infective complications, neurotoxicity, and chemical meningitis.
How should intraocular lymphoma be treated?
The eye is another reservoir for PCNSL tumor cells. Chemotherapy efficacy depends on intraocular pharmacokinetics, which are not well understood for most cytostatics. Systemic administration of MTX and ara-C can yield therapeutic drug levels in the intraocular fluids and clinical responses have been documented; however, drug concentrations in vitreous humor are unpredictable and intraocular relapse is common.54 For these reasons, I treat patients with PCNSL and intraocular disease with HD-MTX–based chemotherapy followed by WBRT and ocular irradiation, which results in better disease control.55 Some authors are investigating the role of direct intravitreal injection of cytostatics. Intravitreal MTX is highly active (remission in 100% of treated eyes) but does not affect OS and is associated with important side effects in 73% of eyes and significant deterioration of visual acuity in 27% of patients.56,57 Among the other drugs examined, intravitreal rituximab appears safe and active.58
Is HDC/ASCT indicated?
Overall, high-dose chemotherapy with autologous stem cell transplantation (HDC/ASCT) is used as a dose intensification treatment to overcome drug resistance. In PCNSL, HDC/ASCT should improve CNS drug bioavailability and replace WBRT to reduce neurotoxicity. In patients with relapsed or refractory PCNSL (Table 2), HDC/ASCT has been associated with 60% CRR, a 2-year OS of 45%, with treatment-related mortality of 16% and severe neurotoxicity in 12% of cases.59 First-line HDC/ASCT was investigated in a few small phase 2 trials (Table 2), suggesting a curative effect in young patients.60 However, efficacy data are controversial because of different induction and conditioning combinations. Cumulative results suggest that HD-MTX–based polychemotherapy induction is more active than monochemotherapy, while intensification with HD–ara-C, alone or in combination, was useful as mobilizing regimen but did not improve response rates. Among conditioning regimens, those containing thiotepa seem to be more effective but more toxic, in particular, the busulfan-thiotepa combination.60 BEAM (carmustine, etoposide, cytarabine, and melphalan) regimen, which was chosen because widely used for other aggressive lymphomas, was ineffective in PCNSL, with median EFS of 9.3 months.61 I believe the discrepancies in effectiveness between BEAM and thiotepa-based regimens may be related to the different capability of drugs to cross the BBB. In fact, busulfan, thiotepa, and carmustine (BCNU) exhibit excellent CNS penetration, with cerebrospinal fluid levels exceeding 50%-80% serum levels, while CNS penetration rates of agents used in BEAM range from 5%-22%.62 Interestingly, a small phase 2 study suggested that HDC/ASCT could replace consolidation radiotherapy, with a 3-year OS of 77% (Table 2, last line). However, the real impact of HDC/ASCT on neurocognitive functions was not defined because treated patients were not prospectively assessed with adequate neuropsychologic tests. At our institution, we do not use HDC/ASCT as part of first-line therapy because it remains an experimental approach; in routine practice, we use HDC/ASCT with a BCNU-thiotepa conditioning combination as part of salvage treatment for selected patients. I am convinced that the comparison between WBRT and HDC/ASCT in 2 ongoing randomized trials (NCT01011920; NCT00863460) will establish the most effective and better-tolerated strategy to consolidate response to primary chemotherapy.
Which chemotherapy for elderly patients?
Age has a profound influence on prognosis and treatment choices in PCNSL.28,29 A large proportion of PCNSL patients is more than 60 years old and is still treated with radiotherapy alone, with disappointing results.63,64 The addition of HD-MTX–based chemotherapy resulted in improved outcome in selected elderly patients (Table 3). As many others, I believe the interpretation of these results is biased because “the elderly patient” has not been clearly defined in PCNSL. With a few exceptions (Table 3), studies focused on the “elderly” include patients with a median age ≤ 70 years, with the lowest age oscillating between 54 and 60 years, which suggests important selection biases because of the inclusion of several “young” patients. In my opinion, the age upper limit currently used for clinical trials (70-75 years old for conventional-dose combinations or 70 in HDC/ASCT trials) represents an acceptable cutoff point to define elderly patients.
I am for treating all elderly patients with primary chemotherapy and I choose a chemotherapy regimen on the basis of PS and comorbidity. Despite common perception that HD-MTX is too toxic for the elderly, some studies suggest that older patients can tolerate this therapy, provided their renal and liver functions are preserved (Figure 4).65,66 MTX 1-3 g/m2 in combination with alkylating agents resulted in median survival ranging from 14 to 36 months (Table 3), but median PFS was usually disappointing, often requiring salvage WBRT to prolong disease control.67,68 However, this approach is associated with severe neurocognitive decline, which, added to the lack of safe and active salvage therapies, strongly condition outcome in elderly patients.
Is there a role for BBB disruption and intra-arterial chemotherapy?
Reversible BBB disruption by intra-arterial infusion of mannitol followed by intra-arterial chemotherapy aims to increase drug concentrations in the lymphoma-infiltrated brain, with particular value in the delivery of agents unlikely to traverse the BBB.69 In institutions with adequate expertise, this strategy was associated with a 58% CRR, a 5-year PFS of 31% and acceptable morbidity and neurotoxicity.69 I do not expect that BBB disruption and intra-arterial chemotherapy will be used worldwide in the next years because its efficacy is similar to that of conventional treatments but it is a procedurally intensive treatment, requiring monthly intravascular interventions under general anesthesia over the course of 1 year.
Radiotherapy
PCNSL is a radiosensitive tumor and radiotherapy has been the standard treatment for decades. In these patients, the whole brain should be irradiated because of the diffuse infiltrative nature of PCNSL and the inclusion of the eyes within the radiation volume is suggested.55 Focal brain radiotherapy attempts resulted in high recurrence rates. Although microscopic cerebrospinal fluid dissemination is common, craniospinal irradiation does not confer additional survival benefit and is associated with significant morbidity.7 Most trials have used conventional photons font and standard fractionation. As an exception, a RTOG (Radiation Therapy Oncology Group) trial failed to show a clear benefit when hyperfractionated WBRT was used.70 WBRT and tumor bed doses are variable in published trials, these parameters depending on the use of radiotherapy as exclusive or complementary treatment.
Who are the best candidates for radiation therapy alone?
WBRT alone is rarely curative in PCNSL patients because response is usually short-lived, with median survivals ranging from 10 to 18 months.71 The optimal dose of WBRT is controversial, but a dose of 40-50 Gy has been suggested, resulting in a 19% CRR. Doses > 50 Gy and the addition of a boost are associated with increased risk of neurotoxicity without further efficacy.71 WBRT 40-50 Gy appears advisable as exclusive treatment when chemotherapy is contraindicated (Figure 4). Encouraging results were obtained with steroid maintenance after primary WBRT,72 but confirmatory studies are needed.
As exclusive treatment, radiotherapy plays a palliative role in patients with cerebrospinal fluid dissemination. Conversely, it is a curative strategy in indolent lymphomas (13% of all PCNSLs3 ). Patients with marginal zone, small lymphocytic, or lymphoplasmacytic lymphoma, mostly arising in the meninges, have excellent long-term prognosis with local therapy alone (Figure 4) and consolidation radiotherapy may be unnecessary after complete resection. Experience with HD-MTX suggests modest activity.73 Isolated CNS plasmacytoma and Hodgkin lymphoma are other suitable candidates for radiotherapy alone. Plasmacytoma can be successfully managed with involved field irradiation with 50 Gy, preceded by chemotherapy in patients with large lesions.74 WBRT 35-45 Gy followed by a 5-15 Gy boost was proposed for CNS Hodgkin lymphoma, with a median OS from CNS involvement of 44 months.75
Is consolidation radiotherapy really needed?
Consolidation after HD-MTX–based chemotherapy represents the best role for radiotherapy in patients with PCNSL (Figure 4). However, this strategy is associated with disabling neurotoxicity, with a cumulative 25%-35% incidence at 5 years and related 30% mortality.76 Long-term impairment in the areas of attention, executive function, memory, and psychomotor speed are the most commonly reported. The mechanisms by which treatment produces CNS damage are unknown, but demyelination, necrosis, and microcavitary changes have been described. Neurotoxicity in PCNSL patients has not been clearly defined because it is usually assessed in small series and rarely in prospective trials. Importantly, a panel of neuropsychologic tests to assess, quantify and follow-up treatment-related neurologic deterioration in PCNSL patients was recently established.77 I expect its wide use will allow better definition of this severe complication both in prospective trials and everyday practice.
To avoid postchemotherapy, WBRT was proposed as the main strategy to reduce neurotoxicity risk. Feasibility and impact of this approach should be analyzed separately in subgroups of patients divided according to response degree after chemotherapy. Complementary WBRT in patients with residual disease after chemotherapy is unavoidable in routine practice because of the lack of valid alternatives. The small group of available active drugs has shown a significantly lower activity in PCNSL patients unresponsive to upfront chemotherapy; only HDC/ASCT has shown some activity in these patients,78 but this remains an experimental approach. In my opinion, the use of any second-line chemotherapy in not irradiated patients should be confined to prospective trials. In everyday practice, I strongly recommend WBRT 40-45 Gy for patients with residual disease after primary chemotherapy.
Recommendations on consolidation WBRT in patients in complete remission (CR) after chemotherapy are less clear. Some small experiences, mostly on elderly patients, suggest that avoiding consolidation WBRT is feasible and results are similar to those obtained with chemoradiation combinations.79 A CALGB (Cancer and Leukemia Group B) phase 2 trial recently reported in abstract form,44 assessed a combination of HD-MTX, temozolomide, and rituximab followed by consolidation with HD-araC and HD-VP16 without WBRT in 46 patients, with a 3-year PFS and OS of 50% and 67%, respectively, a single toxic death and no evidence for significant iatrogenic neurotoxicity. These encouraging results, suggesting that consolidation WBRT can be deferred until relapse, deserve to be assessed in future randomized trials. In a recent randomized trial,80 551 patients treated with HD-MTX–based chemotherapy were randomly allocated to receive WBRT 45 Gy versus observation in the case of CR after chemotherapy or versus HD-araC in the case of partial or no response. The results indicate that consolidation WBRT is associated with a significantly better PFS (median = 18 vs 12 months), but does not change OS (median = 32 vs 37 months). Unfortunately, this trial is fraught with design and execution flaws resulting in important interpretation biases and unreliable conclusions.81,82 In fact, this trial exhibits major protocol violations in 30% of patients, biased analyses of these violations, evident unbalanced comparisons resulting from randomization caveats in ∼ 20% of patients, inconsistent data on iatrogenic neurotoxicity, and 10% of patients lost to follow-up. Moreover, it has low statistical power (60%) and failed to prove the primary hypothesis. As many others,81,82 I believe this trial does not provide reliable conclusions on the role of consolidation WBRT. In my opinion, the optimization of radiation parameters is a valid alternative to radiation withdrawal in PCNSL. Two recent studies suggested that WBRT dose reduction to 23-30 Gy in patients in CR after chemotherapy is associated with similar outcomes to receiving higher radiation doses, with better neurotolerability.83,84 These observations deserve to be addressed in multicenter prospective trials, with standardized neuropsychologic assessment.
In our institution, we discuss with selected patients the pros and cons of using consolidation WBRT or alternatives in everyday practice (Figure 4). I strongly encourage the enrolment of patients in prospective trials comparing consolidation WBRT with experimental approaches because these studies will furnish important data useful to minimize neurotoxicity. Hopefully, I expect that future clinical, biologic, and technologic investigations will provide relevant information on how to maintain neuropsychologic performance in these patients.
Salvage treatment
A variety of salvage therapies was successfully used in selected subgroups of patients with recurrent or refractory PCNSL (Table 4). Although these therapies may be used in routine practice, I am convinced that patients with failed disease must be entered into phase 1/2 trials assessing new drugs and combinations. Although the amount of trials assessing first-line treatments is increasing and failure rates in those trials range between 40% and 70%, the number of reported studies on salvage therapy remains negligible. This is because of the hasty and aggressive course of relapsing PCNSL that produces a drastic PS impairment preventing physicians from enrolling patients in prospective trials and, sometimes, from recommending any treatment.
In routine practice, salvage treatment should be chosen on the basis of the patient's age, PS, the site of relapse, prior therapy, and duration of previous response. At our institution, we give WBRT to patients who experienced failure after chemotherapy alone (Table 4). This strategy is associated with a median survival after relapse of 16 months, but with increased risk of neurotoxicity.67,68 In a few studies, previously nonirradiated, relapsing patients have been treated with salvage chemotherapy to improve disease control while reducing neurotoxicity; reinduction with HD-MTX is an example.85 In my opinion, salvage WBRT should be offered to previously nonirradiated, relapsing patients considering that radiotherapy is more active than most salvage chemotherapies and that the majority of cytostatics used in these combinations were not previously addressed in ad hoc prospective trials.
Single drugs or chemotherapy combinations as well as HDC/ASCT have produced encouraging results in patients relapsing after chemoradiation therapy (Table 4). Isolated systemic (extra-CNS) dissemination (3%-7% of failures3 ) seems to be associated with a significantly better outcome with respect to CNS relapses, in particular if treated with conventional antilymphoma chemotherapy.86
Future perspectives
I expect that future therapeutic progress in PCNSL will be mainly based on the expansion of molecular and biologic knowledge, the improvement of diagnostic sensitivity and specificity, the conduction of well-designed prospective trials, and the prevention of iatrogenic neurotoxicity. All these goals will require multidisciplinary and international efforts. Laboratory research will allow us to better understand important tumor mechanisms and to identify new therapeutic targets. Molecular and biologic studies will require adequate amounts of freshly stored biologic samples; neurosurgeons should be encouraged to consider investigational purposes other than diagnostic suitability and sequel risk when making a decision on sample size. The use of new, more expensive laboratory techniques for research in this “orphan” malignancy will require additional funding from scientific institutions and societies.
Modern functional neuroimaging will help us to establish a suspicion of PCNSL earlier, resulting in timely treatment and less CNS damage. Conducting well-designed trials is key to improve therapeutic efficacy. IELSG no. 2040 and G-PCNSL-GS180 studies opened the era of randomized trials in PCNSL and both randomized trials and phase 2 trials testing new drugs in relapsing patients should be encouraged. All these approaches should be associated with the development of strategies aimed to prevent iatrogenic neurotoxicity given the importance and fragility of organs where this lymphoma arises.
Acknowledgments
I am thankful to the Colleagues and Friends of the International PCNSL Collaborative Group (IPCG). The continuous update and discussion with them led me to improve my own knowledge on this demanding malignancy. Our debates on new concepts and clinical trials, even if sometimes a little spicy, are the fertile background for future improvements in the management of PCNSL patients.
I appreciate the excellent support of scientists and clinicians from the San Raffaele Scientific Institute (Milan, Italy), in particular: Silvia Govi, Silvia Mappa, Marta Bruno Ventre and Emerenziana Marturano (Unit of Lymphoid Malignancies), Michele Reni (Medical Oncology Unit), Maurilio Ponzoni, Maria Rosa Terreni and Claudio Doglioni (Pathology Unit), Marco Foppoli and Federico Caligaris-Cappio (Internal Medicine Unit), Fabio Ciceri (BMT and Hematology Unit), Anna Chiara (Radiotherapy Unit), Alberto Franzin and Piero Picozzi (Neurosurgery Unit), Letterio Politi and Andrea Falini (Neuroradiology Unit), Giulio Modorati (Ophthalmology Unit), Giulio Truci (Neurology Unit), and coworkers.
Authorship
Contribution: A.J.M.F. wrote the entire manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Andrés J. M. Ferreri, MD, Unit of Lymphoid Malignancies, Department of Onco-Hematology, San Raffaele Scientific Institute, Via Olgettina 60, 20132-Milan, Italy; e-mail: andres.ferreri@hsr.it.