Abstract
Viral and fungal infections remain a leading cause of mortality in patients after hematopoietic stem cell transplantation (HSCT). Adoptive transfer of multipathogen-specific T cells is promising in restoring immunity and thereby preventing and treating infections, but approaches are currently limited because of time-consuming and laborious procedures. Therefore, we investigated a new strategy to simultaneously select T cells specific for viral and fungal pathogens based on activation-dependent expression of CD154. Single- and multipathogen-specific T-cell lines with high specificity for adenovirus (AdV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), Candida albicans, and/or Aspergillus fumigatus could be readily generated within 14 days irrespective of the precursor frequency. The T-cell lines responded reproducibly to endogenously processed antigen and specifically proliferated upon antigenic stimulation. Although isolation based on CD154 favors enrichment of CD4+ T cells, AdV-, EBV- and CMV-specific CD8+ T cells could be expanded and demonstrated lysis of target cells. Conversely, T cell–mediated alloreactivity was almost abrogated compared with the starting fraction. This selection and/or expansion strategy may form the basis for future adoptive immunotherapy trials in patients at risk for multiple infections and may be translated to other antigens.
Introduction
Despite substantial improvements in treatment and prophylaxis, infectious complications remain a leading cause of morbidity and mortality after hematopoietic stem cell transplantation (HSCT).1 The development and application of immunotherapeutic strategies such as T-cell transfer is aimed at integrating or replacing anti-infective therapy to reduce infectious complications, drug resistance, and toxicity. The efficacy and safety of adoptive T-cell therapy targeting posttransplantation viral or fungal infections in HSCT recipients has already been successfully demonstrated.2-4 To minimize therapeutic intervention and to maximize the clinical efficacy of T-cell transfer, expansion protocols have been established to generate multipathogen-specific T-cell lines with simultaneous antiviral and antileukemic specificity.5,6 However, the generation of these T-cell lines is to date laborious and time-consuming, taking 4-6 weeks for cell expansion, and necessitates repeated restimulation with retrovirally transduced autologous antigen-presenting cells.6 More rapid approaches have been developed recently based on cytokine secretion. One example is the IFN-γ capture assay, which is restricted to antigens with moderate- to high-memory T-cell frequencies in the peripheral blood, such as cytomegalovirus (CMV) and adenovirus (AdV).7,8 To increase the sensitivity for the isolation of rare, pathogen-specific memory cells, other T-cell–activation markers that enable the capture of antigen-specific T cells irrespective of cytokine production may be more suitable. Different T-cell surface molecules that are selectively expressed or strongly up-regulated after T-cell activation, including CD25, CD69, CD71, CD134, CD137, and CD154, could be similarly useful for the selection of antigen-specific T cells.9-15 CD154, which is transiently expressed on activated CD4+ T cells and to a lesser extent on activated CD8+ T cells after antigen contact, has been shown recently to be a promising candidate for the selection of pathogen-specific T cells because of its high specificity and sensitivity.10,16
Posttransplantation infections are caused by viral and fungal pathogens such as AdV, CMV, Epstein-Barr virus (EBV), Aspergillus fumigatus, and Candida albicans,17-19 which harbor various memory T-cell pools in the peripheral blood. We therefore investigated whether a new isolation strategy based on activation-dependent expression of CD154 can enrich and expand CD4+ and CD8+ T cells specific for these viral and fungal pathogens within a short time period. Because CMV-specific T cells might dominate multipathogen-specific cultures due to comparatively higher precursor frequencies,5 and because the seropositivity of a representative population ranges only between 40%-50%,20 we first focused on the 4 pathogens AdV, EBV, A fumigatus, and C albicans. These pathogens are highly prevalent in HSCT recipients, and the majority of HSCT donors have been already been exposed to them and therefore harbor pathogen-specific memory T cells. We expanded our investigation by including CMV as fifth antigen in our protocol.
Methods
Peptides and AdV lysate
Peptide libraries covering the complete sequences of EBV latent membrane protein 2 (LMP2), C albicans mannose protein 65 (MP65), AdV hexon protein, and CMV pp65 protein consisting of 15-mer peptides overlapping by 11 amino acids were purchased from JPT Peptide Technologies.
The A fumigatus Crf1 15-mer peptide p41 (FHTYTIDWTKDAVTW) was obtained from Proimmune. Adenovirus lysate was purchased from Virion/Serion.
Fungal strains and generation of fungal extract and inactivated fungus
A fumigatus strain D141 and C albicans strain SC5314 were kindly provided by Prof Dr Sven Krappmann and Prof Joachim Morschhäuser, respectively (both of the University of Wuerzburg, Wuerzburg, Germany). Fungal extracts of A fumigatus were generated as described previously.21 Protein content was measured with the Bradford protein assay (Perbio Science) according to the manufacturer's instructions. For the generation of inactivated fungus, C albicans was inoculated in minimal medium with glucose and nitrate22 and cultured for 24 hours. Fungi were inactivated by incubation with 70% ethanol for 30 minutes. Ethanol-inactivated fungi and extracts were tested for sterility by subculturing on Sabouraud dextrose agar for 3 days.
Blood donors and cell culture
Blood was obtained after informed consent from 7 healthy donors in accordance with the Declaration of Helsinki. All studies were approved by the University of Wuerzburg institutional review board. Donors were typed for HLA class I and HLA-DRB1 alleles. Only donors with HLA-DRB1*03+ (n = 1), HLA-DRB1*04+(n = 5), and HLA-DRB1*13+(n = 1) were included because these alleles are able to present the A fumigatus p41 peptide.23 PBMCs and dendritic cells (DCs) were generated according to previously published protocols.8 DCs were either used immature or were matured by the addition of 25 ng/mL of TNF-α, 5 ng/mL of IL-1β, 10 ng/mL of IL-6 (all R&D Systems), and 1 μg/mL of PGE2 (Pharmacia) for 24 hours.
Generation and expansion of antigen-specific T-cell lines
Antigen-specific T-cell lines were generated by overnight incubation of 5-8 × 107 PBMCs in 7.5-12 mL of medium in 6-well culture plates in the presence of antigen and 1 μg/mL of anti-CD40 antibody (Miltenyi Biotec). For the single T-cell lines, the cultures were either stimulated with 0.5 μg/mL of the AdV peptide pool, 0.5 μg/mL of the EBV LMP2 peptide pool, 5 μg/mL of the A fumigatus Crf1 peptide p41, 0.5 μg/mL of the C albicans MP65 peptide pool, or 0.25 μg/mL of the CMV pp65 peptide pool. For the multipathogen-specific T-cell lines, PBMCs were stimulated with the same amount of peptide as for the single lines, with the exception of the EBV LMP2 peptide pool, which was increased to 2 μg/mL because preceding experiments showed lower expansion of antigen-specific T cells. After 16 hours, antigen-specific cells were enriched by the selection of CD154+ cells via magnetic cell separation (Miltenyi Biotec). Up to 2 × 105 CD154+ cells were cultured in the presence of 50:1 γ-irradiated (30 cGy) autologous feeder cells and 5 U/mL of IL-2 (Proleukin; Chiron) for 7 days. From days 8-14, the cultures were supplemented with 10 ng/mL IL-7 and IL-15. Lymphocyte cultures were supplemented with cytokines every other day and culture medium replenished as needed.
Immunophenotyping and tetramer staining
Antigen-stimulated T cells were stained before and after selection with monoclonal antibodies to CD4 and CD8 (Becton Dickinson). Samples were analyzed using FACSCantoII (BD Biosciences) and FlowJo 7.1.3 software (TreeStar).
The phycoerythrin-labeled (Beckman Coulter) A fumigatus Crf1-specific MHC class II tetramer DRB1*0401 FHTYTIDWTKDAVTW was used according to the manufacturer's instructions.
IFN-γ ELISPOT and ELISA assays and ICC assay
Precursor frequencies of antigen-specific memory cells in PBMCs were analyzed by IFN-γ ELISPOT assay after overnight stimulation of 1 × 104 to 2 × 105 cells/well with 0.5 μg/mL of the AdV hexon protein peptide pool, 0.5 μg/mL of the EBV LMP2 peptide pool, 5 μg/mL of the A fumigatus Crf1 peptide p41 pool, or 0.5 μg/mL of the C albicans MP65 peptide pool. Frequencies of isolated CD4+ and CD8+ T cells (Miltenyi Biotec) and expanded antigen-specific cells were analyzed by IFN-γ ELISPOT assay after restimulation with mature antigen-pulsed autologous DCs at a responder-to-stimulator ratio of 10:1. ELISPOT was performed according to the manufacturer's instructions. IFN-γ spots were counted using an ELISPOT reader (Cellular Technologies). The number of spot forming cells (SFCs) per 2 × 105 cells was calculated from duplicates. Reactivity of T-cell lines to endogenously processed antigen was analyzed by IFN-γ ELISA8 after restimulation with autologous immature DCs incubated overnight with 5 μg/mL of AdV lysate, 20 μg/mL of A fumigatus cell extract, inactivated C albicans yeast (MOI 3), or partially matched allogeneic EBV-lymphoblastoid cell lines (LCLs; kindly provided by Dr Patricia Comoli, University Pavia, Italy and Dr Inge Jedema, University Medical Center, Leiden, The Netherlands) or CMV pp65-transduced LCLs at a responder-to-stimulator ratio of 5:1. Intracellular cytokine (ICC) staining was performed as described previously.8 T-cell lines were stimulated with autologous peptide-pulsed DCs at a responder-to-stimulator ratio of 10:1 with the same concentration of antigens.
Analysis of cell proliferation by [3H]-thymidine incorporation
Antigen-specific T-cell lines (1 × 105 cells/well) were restimulated on day 21 after resting for 5 days without cytokines with mature autologous DCs pulsed with the same amounts of antigens used for generation of the T-cell lines at a responder-to-stimulator ratio of 10:1 for 72 hours. DNA synthesis was assayed by adding 1 μCi (0.037 MBq) of [3H]-thymidine (Amersham) per well during the last 18 hours of culture. The cells were harvested on glass filter paper and the counts per minute (cpm) determined using a liquid scintillation counter.
Cytotoxicity assay
The 51Cr-release assay was performed as described previously.24 In brief, 5 × 105 partially matched LCLs pulsed with 0.5 μg/mL of the AdV hexon protein peptide pool, 0.5 μg/mL of the EBV LMP2 peptide pool, or mismatched or CMV pp65-transduced LCLs were used as target cells and pulsed with 100 μCi Na2[51Cr]O4 for 1 hour. Antigen-specific T cells were added in effector-to-target ratios of 5:1, 10:1, and 20:1 in triplicate, and [51Cr] release was determined in the culture supernatant after 4 hours of incubation. Maximum release was determined by lysis of target cells with 2% Triton X-100. The percentage of specific lysis was determined as follows: ([experimental release − spontaneous release]/[maximum release − spontaneous release]) × 100.
Mixed lymphocyte reaction
Autologous PBMCs and antigen-specific T-cell lines from day 14 were cultured with autologous and 2 different third-party DCs (responder-to-stimulator ratio, 5:1) for 96 hours, and [3H]-thymidine incorporation was measured as described in “Analysis of cell proliferation by [3H]-thymidine incorporation.”
Statistical analysis
Analyses were conducted using Prism 5.0 program (GraphPad). The Student t test or ANOVA and the Bonferroni test were used to determine the statistical significance (P). Data of individual donors are shown as representative experiments or means with SD. Combined data of different donors are given as median with range.
Results
The frequencies of virus-specific memory T cells are higher than of fungus-specific T cells
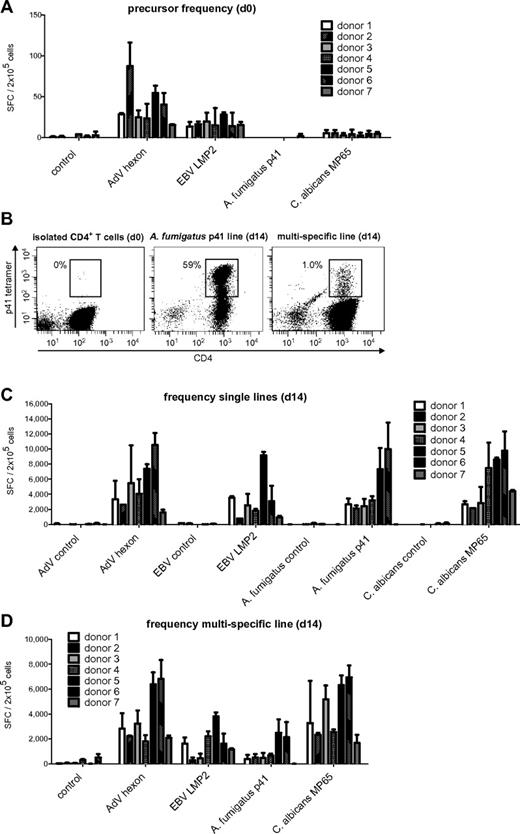
To estimate the pool of memory T cells specific for AdV, EBV, C albicans, and A fumigatus, PBMCs of 7 healthy donors were stimulated with previously described, commercially available, broadly immunogenic peptide pools for AdV hexon protein,7,25,26 C albicans MP65,27-29 and subdominant EBV LMP2,24,30 and the frequency of antigen-specific T cells was assessed by IFN-γ ELISPOT assay. For A fumigatus, we used a single peptide of the cell wall glucanase Crf131 that was recently identified by our group.23 The frequency of virus-specific T cells was in general higher than that of fungus-specific T cells (Figure 1A). The median IFN-γ response of T cells specific for AdV was 28.3 SFCs/2 × 105 cells; for EBV, 15.5 SFCs/2 × 105 cells, for C albicans, 4.3 SFCs/2 × 105 cells; and for A fumigatus, it was undetectable. To assess whether the observed cytokine response was mediated by CD4+ or CD8+ T cells, isolated CD4+ and CD8+ T cells were stimulated with antigen-pulsed DCs. AdV hexon and C albicans MP65 peptide pools elicited a predominant CD4+ T-cell response, whereas the EBV LMP2 peptide pool favored a CD8+ T-cell response (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A fumigatus p41 peptide-specific T cells were not detectable in isolated CD4+ or CD8+ T cells. This low precursor frequency was verified by tetramer staining, because we identified at most 1-2 HLA-DRB1*0401/p41 tetramer-positive CD4+ T-lymphocytes per 105 CD4+ T cells in HLA-DRB1*0401-positive donors (Figure 1B).
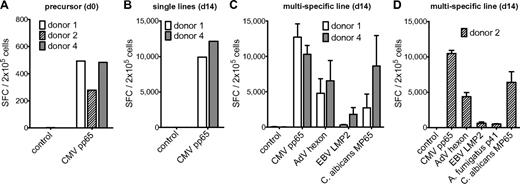
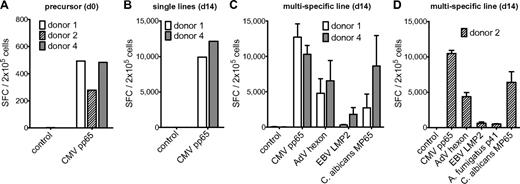
Memory T-cell frequencies for AdV hexon protein, EBV LMP2 protein, A fumigatus p41 peptide, and C albicans MP65 protein and expansion of antigen-specific cells. (A) Precursor frequencies of T cells specific for the AdV hexon peptide pool, the EBV LMP2 peptide pool, the A fumigatus Crf1 peptide p41, and the C albicans MP65 peptide pool in PBMCs of 7 different donors assessed by IFN-γ ELISPOT assay. (B) Quantification of A fumigatus p41–specific T cells by HLA DRB1*0401–restricted phycoerythrin-labeled tetramer staining in isolated CD4+ T cells, A fumigatus–specific single-pathogen T-cell lines, and multipathogen-specific lines 14 days after expansion. Depicted are 1 × 106 T cells in isolated CD4+ T cells and 1.5 × 104 T cells for single A fumigatus- and multipathogen-specific T-cell lines (representative example, n = 6). (C) Frequencies of T cells specific for the AdV hexon peptide pool, the EBV LMP2 peptide pool, the A fumigatus Crf1 peptide p41, and the C albicans MP65 peptide pool after 14 days of expansion in single cultures and (D) after 14 days of expansion in multipathogen-specific cultures. Frequencies (A,C,D) were determined by IFN-γ ELISPOT assay and are expressed as SFCs/2 × 105 cells (n ≥ 2).
Memory T-cell frequencies for AdV hexon protein, EBV LMP2 protein, A fumigatus p41 peptide, and C albicans MP65 protein and expansion of antigen-specific cells. (A) Precursor frequencies of T cells specific for the AdV hexon peptide pool, the EBV LMP2 peptide pool, the A fumigatus Crf1 peptide p41, and the C albicans MP65 peptide pool in PBMCs of 7 different donors assessed by IFN-γ ELISPOT assay. (B) Quantification of A fumigatus p41–specific T cells by HLA DRB1*0401–restricted phycoerythrin-labeled tetramer staining in isolated CD4+ T cells, A fumigatus–specific single-pathogen T-cell lines, and multipathogen-specific lines 14 days after expansion. Depicted are 1 × 106 T cells in isolated CD4+ T cells and 1.5 × 104 T cells for single A fumigatus- and multipathogen-specific T-cell lines (representative example, n = 6). (C) Frequencies of T cells specific for the AdV hexon peptide pool, the EBV LMP2 peptide pool, the A fumigatus Crf1 peptide p41, and the C albicans MP65 peptide pool after 14 days of expansion in single cultures and (D) after 14 days of expansion in multipathogen-specific cultures. Frequencies (A,C,D) were determined by IFN-γ ELISPOT assay and are expressed as SFCs/2 × 105 cells (n ≥ 2).
Virus- and fungus-specific CD4+ and CD8+ T cells can be isolated based on activation-dependent expression of CD154
To determine the optimal time at which to select antigen-specific T cells, we investigated the dynamics of intracellular and surface expression of CD154 after stimulation of donors with high precursor frequencies for the A fumigatus p41 or the CMV pp65 peptide pools. Previous data with mitogenic stimuli showed an up-regulation of CD154 on the surface of T cells 6 hours after activation that could be stabilized by the addition of CD40-blocking antibody.10 Surface expression of CD154 was not detectable upon stimulation with either peptide, whereas intracellular staining showed an increasing up-regulation of CD154 after 12-16 hours by both antigens with additional CD40-blocking antibody (data not shown). Based on these results, we performed CD154 selection 12-16 hours after antigenic stimulation.
T cells specific for all pathogens could be enriched from PBMCs of all 7 healthy donors. In median, 0.88% (range 0.35%-1.33%) of 5-9 × 107 PBMCs were isolated (Table 1). The purity of the isolated cells as determined by antibiotin antibody was only 8%-15% (data not shown). The phenotype was predominately CD4+ (median 93%), which is in contrast to the unselected populations that contained 50%-60% CD4+ and 15%-30% CD8+ T cells (data not shown).
Virus- and fungus-specific CD4+ and CD8+ memory T cells can be expanded and enriched to high antigen specificity within 14 days irrespective of the precursor frequency
The cell population isolated by CD154 expression could be expanded 3- to 145-fold (median, 62.5-fold) depending on the antigen and donor (Table 1). Although isolation of CD154+ cells greatly favors enrichment of CD4+ T cells, a small number of CD8+ T cells was simultaneously expanded depending on the type of antigen (median for AdV, 1%; EBV, 9.5%; A fumigatus, 1.25%; and C albicans, 0.5%; data not shown).
The expanded T-cell lines of all donors showed high specificity for AdV, EBV, and C albicans. A fumigatus p41-specific T-cell lines could be generated from 6 of 7 donors. The median IFN-γ response on day 14 was 4315 SFCs/2 × 105 cells for AdV hexon, 2545 SFCs/2 × 105 cells for EBV LMP2, 2650 SFCs/2 × 105 cells for A fumigatus p41, and 4265 SFCs/2 × 105 cells for C albicans MP65 (Figure 1C).
Based on the frequency of antigen-specific T cells in the starting fraction and after expansion on day 14, the antigen-specific expansion of each T-cell line was calculated. The median frequency of AdV-specific T cells increased by 152-fold, of EBV-specific T cells by 164-fold, and of C albicans–specific T cells by 992-fold. Because A fumigatus–specific T cells were undetectable by IFN-γ ELISPOT assay, we used the tetramer to calculate the expansion rate. Tetramer staining showed 40% (range 25%-63%) of A fumigatus p41 peptide-specific CD4+ T cells after 14 days of expansion (Figure 1B), which indicates an expansion rate of approximately 20 000-fold.
Virus- and fungus-specific memory T cells can be simultaneously expanded in a single T-cell culture
We tested the efficacy of CD154-based enrichment for the generation of multipathogen-specific T-cell lines reactive to the 2 viral and the 2 fungal pathogens. PBMCs were stimulated with a combination of the same amount of peptide pools except for LMP2, which was increased to 2 μg/mL because preceding experiments showed lower expansion of antigen-specific T cells. The cell count of the isolated CD154+ fraction (median, 0.98%) and median total cell expansion (68-fold) were not significantly different for multipathogen-specific T-cell lines compared with T-cell lines with single specificity (Table 1). Similarly, the ratio of CD4+ T cells (median 91%) to CD8+ T cells (median 3%) in multipathogen-specific T-cell lines was comparable to cultures with single specificity (data not shown).
All multipathogen-specific T-cell lines contained T cells specific for all 4 antigens, except for donor 7, who did not respond to stimulation with A fumigatus p41 peptide (as shown previously in the single culture). However, there was a notable decrease in the frequencies of antigen-specific T cells in multipathogen-specific cultures compared with single lines. Median of antigen-specific T cells: AdV 2830 vs 4315 SFCs/2 × 105 cells (P < .05), EBV 1620 vs 2545 SFCs/2 × 105 cells (not significant), A fumigatus 510 vs 2650 SFCs/2 × 105 cells (P < .001), and C albicans 3270-4265 SFCs/2 × 105 cells (P < .05; Figure 1D). The median tetramer positivity was similarly lower with only 1%-2% for the A fumigatus HLA-DRB1*0401/p41 tetramer (Figure 1B).
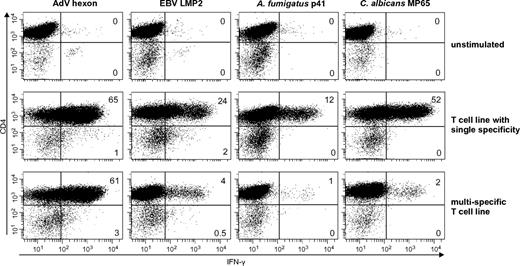
To distinguish if the T-cell response was mediated by CD4+ or CD8+ T cells, we determined the IFN-γ response to stimulation with the respective antigens by intracellular cytokine staining and flow cytometric analysis. Similar to the single antigen-specific T-cell lines, the multipathogen-specific T-cell lines showed a predominant CD4+ IFN-γ response after stimulation with all antigens. Only the viral antigens EBV LMP2 (median CD8+ IFN-γ, 27%; range, 14%-40%) and AdV hexon protein (median CD8+ IFN-γ 42%; range 0%-77%) induced additionally a CD8+ IFN-γ T-cell response (Figure 2).
Functionally active single- and multipathogen-specific CD4+ and CD8+ T cells are enriched after selection and expansion. Percentage of IFN-γ–positive CD4+ T cells after 14 days of enrichment. Cells were stimulated with the respective antigens and assessed by ICC (representative example, n = 4 with ≥ 2 repetitions).
Functionally active single- and multipathogen-specific CD4+ and CD8+ T cells are enriched after selection and expansion. Percentage of IFN-γ–positive CD4+ T cells after 14 days of enrichment. Cells were stimulated with the respective antigens and assessed by ICC (representative example, n = 4 with ≥ 2 repetitions).
Ex vivo–generated single- and multipathogen-specific T-cell lines show antigen-specific proliferation after stimulation and antiviral cytotoxic activity
[3H]-thymidine incorporation was measured to assess whether the ex vivo–generated T-cell lines are able to proliferate upon antigen stimulation (Figure 3). Single-specific T-cell lines of all antigens showed strong proliferation. Multipathogen-specific T-cell lines demonstrated high proliferation for AdV- and C albicans–specific T cells, whereas proliferation in response to restimulation with EBV and A fumigatus was diminished (EBV, P = not significant; A fumigatus, P < .001). In contrast, unpulsed DCs elicited only very weak proliferation, indicating that proliferation is strictly antigen-specific.
Antigen-specific T cells in single- and multipathogen-specific lines specifically proliferate upon antigen contact. Proliferation of T-cell lines specific for AdV hexon, EBV LMP2, A fumigatus p41, and/or C albicans MP65 after restimulation with the peptide pool as determined by [3H]-thymidine incorporation after 72 hours. Medians and range of at least 3 independent experiments performed in triplicate of 3 different donors are shown. Reduction of proliferative potential of multipathogen-specific lines versus single lines was only statistically significant for A fumigatus p41 (P < .001).
Antigen-specific T cells in single- and multipathogen-specific lines specifically proliferate upon antigen contact. Proliferation of T-cell lines specific for AdV hexon, EBV LMP2, A fumigatus p41, and/or C albicans MP65 after restimulation with the peptide pool as determined by [3H]-thymidine incorporation after 72 hours. Medians and range of at least 3 independent experiments performed in triplicate of 3 different donors are shown. Reduction of proliferative potential of multipathogen-specific lines versus single lines was only statistically significant for A fumigatus p41 (P < .001).
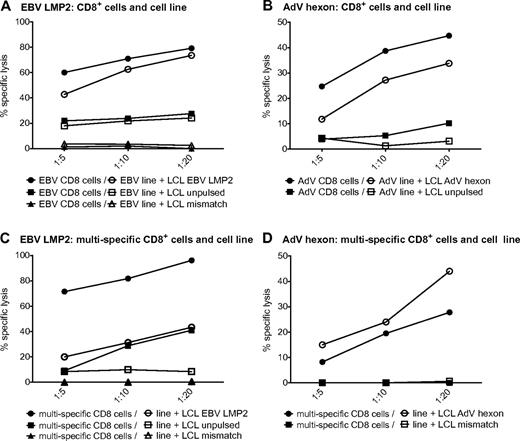
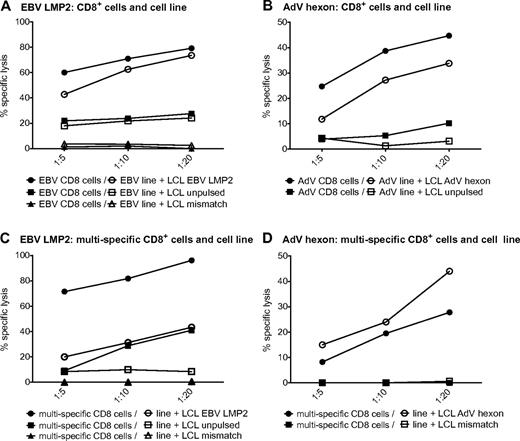
We next investigated whether the single-virus–specific T-cell lines are able to lyse target cells. HLA-A*0201–matched LCLs were pulsed with the respective peptide pools and used as targets. AdV hexon- and EBV LMP2–pulsed targets were efficiently killed by the respective T-cell lines (Figure 4A-B). The EBV LMP2–specific T-cell lines were also able to lyse to a lesser degree the partially matched, unpulsed LCLs (Figure 4A). To investigate whether target cell killing by antigen-specific CD8+ cytotoxic T cells is hampered by their comparatively low frequency in the T-cell lines, we also analyzed target cell lysis by isolated CD8+ T cells of AdV and LMP2 single lines. Killing by isolated CD8+ T cells was similar for EBV and elevated for AdV. These results indicate that the number of antiviral cytotoxic T cells is sufficient in single-pathogen lines (Figure 4A-B). The multipathogen-specific T-cell lines were also able to lyse peptide-pulsed targets, although to a lesser extent than the single lines. Killing by isolated CD8+ T cells was substantially increased for LMP2-pulsed LCLs and partially matched LCLs, indicating that this effect is dependent on the number of EBV-specific CD8+ T cells contained in the T-cell culture (Figure 4C).
Virus-specific T cells in single- and multipathogen-specific cultures efficiently kill target cells. Specific lysis of negative control, partially matched, unpulsed LCLs, or partially matched LCLs pulsed with EBV LMP2 peptide pool by an EBV-specific T-cell line or CD8+ T cells isolated from an EBV-specific cell line (A) or a multipathogen-specific line (C). Specific lysis of negative control or partially matched LCLs pulsed with AdV hexon peptide pool by an AdV-specific T-cell line or CD8+ T cells isolated from an AdV-specific cell line (B) or a multipathogen-specific line (D). Specific lysis was determined with the 51Cr-release assay (representative experiment, n ≥ 2 for each of 2 donors).
Virus-specific T cells in single- and multipathogen-specific cultures efficiently kill target cells. Specific lysis of negative control, partially matched, unpulsed LCLs, or partially matched LCLs pulsed with EBV LMP2 peptide pool by an EBV-specific T-cell line or CD8+ T cells isolated from an EBV-specific cell line (A) or a multipathogen-specific line (C). Specific lysis of negative control or partially matched LCLs pulsed with AdV hexon peptide pool by an AdV-specific T-cell line or CD8+ T cells isolated from an AdV-specific cell line (B) or a multipathogen-specific line (D). Specific lysis was determined with the 51Cr-release assay (representative experiment, n ≥ 2 for each of 2 donors).
Peptide-generated T-cell lines respond to endogenously processed viral and fungal antigen
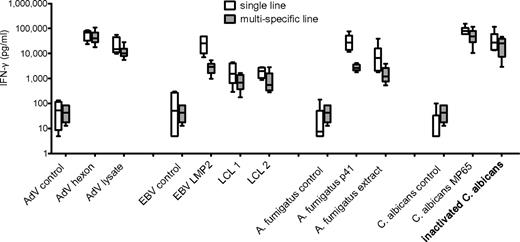
We further investigated the ability of T-cell lines generated by peptide stimulation to recognize and respond to endogenously processed viral and fungal antigens. The T-cell lines were restimulated either with peptide or the natural antigens AdV lysate, 2 different partially HLA-matched EBV-transformed LCLs, A fumigatus cell extract, or ethanol-inactivated C albicans yeast. All T-cell lines responded readily to endogenously processed natural antigens. Although lower responses for naturally processed antigens were determined, no significant differences could be detected either between single versus multipathogen-specific T-cell lines or between stimulation with peptide versus endogenously processed antigen (Figure 5).
Peptide pool–specific T-cell lines respond to endogenously processed antigen. T-cell lines specific for AdV hexon protein, EBV LMP2, A fumigatus p41 peptide, C albicans MP65, or multipathogen-specific lines were restimulated with peptide pool or natural antigen (AdV lysate, 2 different partially matched EBV-transfected LCLs, A fumigatus cell extract, and ethanol-inactivated C albicans) and responses assessed by IFN-γ ELISA. Medians and range of at least 5 independent experiments of 3 different donors are shown. All differences between single- versus multipathogen-specific line and stimulation with peptide versus natural antigen were statistically not significant.
Peptide pool–specific T-cell lines respond to endogenously processed antigen. T-cell lines specific for AdV hexon protein, EBV LMP2, A fumigatus p41 peptide, C albicans MP65, or multipathogen-specific lines were restimulated with peptide pool or natural antigen (AdV lysate, 2 different partially matched EBV-transfected LCLs, A fumigatus cell extract, and ethanol-inactivated C albicans) and responses assessed by IFN-γ ELISA. Medians and range of at least 5 independent experiments of 3 different donors are shown. All differences between single- versus multipathogen-specific line and stimulation with peptide versus natural antigen were statistically not significant.
Alloreactivity of ex vivo–generated T-cell lines to third-party DCs is nearly abrogated
Because adoptive transfer of donor-derived T cells into HSCT recipients may induce GVHD, we assessed the alloreactivity of the virus-, fungus-, and multipathogen-specific T-cell lines. The T-cell lines and autologous PBMCs were exposed to third-party DCs for 96 hours and proliferation was determined by the [3H]-thymidine incorporation assay. In all donors, third-party DCs elicited a strong proliferative response in PBMCs, whereas the proliferative capacity of all antigen-specific T-cell lines was nearly abrogated (median reduction of 99.991%; Figure 6).
Single- and multipathogen-specific T-cell lines show nearly abrogated alloreactivity. Alloreactivity was determined 14 days after expansion with 2 different third-party DCs by [3H]-thymidine incorporation 96 hours after stimulation. Proliferative decrease of T-cell lines versus autologous PBMCs statistically significant for all T-cell lines (P < .001).
Single- and multipathogen-specific T-cell lines show nearly abrogated alloreactivity. Alloreactivity was determined 14 days after expansion with 2 different third-party DCs by [3H]-thymidine incorporation 96 hours after stimulation. Proliferative decrease of T-cell lines versus autologous PBMCs statistically significant for all T-cell lines (P < .001).
High-frequency CMV-specific memory cells can be expanded and do not significantly interfere with the other antigens in multipathogen-specific T-cell cultures
Finally, we investigated whether CMV, one of the most important pathogens causing posttransplantation infections, could be included in the multipathogen-specific T-cell cultures. The precursor frequencies of T cells specific for the CMV pp65 peptide pool were higher than those of the previously investigated antigens (Figure 7A). CMV pp65–specific T cells could be enriched and expanded from PBMCs of 2 healthy HLA-DRB1*04 donors, and the median IFN-γ response on day 14 was 11 025 SFCs/2 × 105 T cells (Figure 7B), which is 2.6- to 4.3-fold higher than for the other antigens tested. Because CMV-specific T cells might prevail in multipathogen-specific cultures because of comparatively higher precursor frequencies,5 we either substituted CMV for A fumigatus or used all 5 antigens simultaneously. The cell count of the isolated CD154+ fraction, the expansion rate, and the ratio of CD4+ T cells to CD8+ T cells were similar to the multipathogen-specific T-cell lines described in “Virus and fungus-specific memory T cells can be simultaneously expanded in a single T-cell culture” (Table 2). In both approaches, all antigens could be readily enriched and expanded. The CMV-specific T cells neither dominated the T-cell cultures nor significantly diminished the frequencies of the other antigens (P > .05 for all antigens; Figure 7C-D); again, the CMV-specific T cells of the single- and multipathogen-specific cultures showed cytotoxic activity against CMV pp65–transfected target cells (supplemental Figure 2) and recognized endogenously processed antigen (data not shown).
Specificity of multipathogen-specific T-cell lines can be extended to CMV. (A) Precursor frequencies of T cells specific for the CMV pp65 peptide pool in PBMCs of 3 different donors assessed by IFN-γ ELISPOT assay. (B) Frequency of T cells specific for the CMV pp65 peptide pool after 14 days of expansion in single cultures. (C) Frequencies of T cells specific for the CMV pp65 peptide pool, the AdV hexon peptide pool, the EBV LMP2 peptide pool, and the C albicans MP65 peptide pool, and (D) in addition A fumigatus Crf1 peptide p41 after 14 days of expansion in multipathogen-specific cultures. Frequencies were determined by IFN-γ ELISPOT assay and are expressed as SFCs/2 × 105 cells (n = 2). The differences in the number of antigen-specific cells after expansion between single- and multipathogen-specific lines were not statistically significant.
Specificity of multipathogen-specific T-cell lines can be extended to CMV. (A) Precursor frequencies of T cells specific for the CMV pp65 peptide pool in PBMCs of 3 different donors assessed by IFN-γ ELISPOT assay. (B) Frequency of T cells specific for the CMV pp65 peptide pool after 14 days of expansion in single cultures. (C) Frequencies of T cells specific for the CMV pp65 peptide pool, the AdV hexon peptide pool, the EBV LMP2 peptide pool, and the C albicans MP65 peptide pool, and (D) in addition A fumigatus Crf1 peptide p41 after 14 days of expansion in multipathogen-specific cultures. Frequencies were determined by IFN-γ ELISPOT assay and are expressed as SFCs/2 × 105 cells (n = 2). The differences in the number of antigen-specific cells after expansion between single- and multipathogen-specific lines were not statistically significant.
Discussion
Adoptive transfer of pathogen-specific T cells has been demonstrated to prevent or ameliorate disease without causing adverse events and, further, to enhance long-term immunity against these pathogens.2,4 Simultaneous targeting of several pathogens with a single T-cell product would facilitate its application, but these approaches have been hindered to date by lengthy and intricate processes. In the present study, we have developed a new strategy based on activation-dependent expression of CD154 that expands memory T cells specific for the most common viral and fungal pathogens causing posttransplantation infections. This protocol allows simultaneous enrichment and expansion of CD4+ and CD8+ T cells specific for AdV, CMV, EBV, A fumigatus, and C albicans in a single T-cell culture in spite of different memory T-cell frequencies. Pathogen-specific memory T cells can be enriched with commercially available antigens within 14 days without the need to genetically modify autologous APCs. We confirmed that the expanded T cells had functional specificity against the different pathogens in single- and multipathogen-specific T-cell cultures, recognized endogenously processed antigen, and nearly abrogated alloreactivity.
For the generation of a T-cell product for adoptive transfer, the procedure should be fast and simple to be applicable in a timely fashion to patients in need. The selection of antigen-specific T cells upon stimulation has been shown to simplify and shorten the generation process. Because the approved clinical-grade IFN-γ secretion assay allows only the selection of T cells with moderate to high precursor frequency,7,8 and because isolation/expansion of A fumigatus–specific T cells was not feasible (data not shown), we searched for another activation marker to select rare antigen-specific T cells. Isolation of antigen-specific T cells based on activation-dependent expression of CD154 enabled us to select and expand T cells sensitively and specifically for 3 viral and 2 fungal pathogens (even in a single culture) within 2 weeks. Depending on the donor, the different T-cell lines showed similar expansion of median 2 to 3 log irrespective of the number of isolated cells. In fact, we were even able to expand A fumigatus peptide–specific CD4+ T cells by approximately 20 000-fold, although precursor frequencies in the peripheral blood were barely detectable with a peptide-specific tetramer. However, the generation of T-cell lines with this high frequency and number of antigen-specific cells was only achieved when the cultures were supplemented with IL-7 and IL-15 after day 7. These cytokines reduce activation-induced cell death of antigen-specific T cells, thereby increasing the frequency and repertoire of responding cells compared with T-cell lines generated in the absence of cytokine.32 Such supplementation seems to be unnecessary in other protocols, probably because of longer expansion periods and/or repeated weekly restimulation, which allows the T cells to expand more slowly, thereby diminishing activation-induced cell death.5 The duration and culture conditions of ex vivo T-cell expansion might also influence the in vivo efficacy and safety of a T-cell product. Extensive culturing of antigen-specific T cells has been shown to efficiently diminish alloreactivity but concurrently may limit the proliferative capacity of the transferred T cells in vivo.8,33,34 Conversely, a short-time expansion protocol could be associated with strong T-cell activation and therefore a higher risk of alloreactivity, leading to the development of GVHD. In the present study, we assessed alloreactivity using a method described previously,8,35 although it remains unclear if the assay used meets the criteria for assessing the loss of alloreactivity in vivo. Nevertheless, we were able to demonstrate that despite using cytokines for expansion, alloreactivity is almost abrogated in the antigen-specific T-cell lines of all donors compared with the starting fraction and, moreover, that all expanded antigen-specific T cells showed high proliferative capacity upon antigen stimulation. These T-cell lines generated by peptide antigen could also efficiently recognize naturally processed viral and fungal antigens, suggesting that the activation and expansion of adoptively transferred T cells in vivo after contact with pathogens is very likely.
Apart from A fumigatus, we used previously described, commercially available peptide pools for AdV hexon protein,7,25,26 CMV pp65,8,36 EBV LMP2,24,30 and C albicans MP65.27-29 Stimulation with peptide pools, including broadly immunogenic and conserved epitopes, seems to be especially important for viral T-cell expansion to recruit effector cells with widely varying precursor frequencies and affinities for the target antigen, which may reduce the risk of viral escape mutants,37 a phenomenon that has so far not been described for fungal pathogens. Immunodominant target antigens have not been described much for A fumigatus. Therefore, we used Crf1,31 a single peptide of the cell wall glucanase recently identified by our group that is presented by at least 3 MHC class II alleles. Crf1 is present in ∼ 60% of the white population and shows high proliferative capacity in oligoclonal TH1 cells.23 These data illustrate that the use of synthetic peptides that can be readily produced under GMP regulations and that have previously been shown to be safe may simplify the generation of clinical-grade, multipathogen-specific T-cell lines.38
To ensure in vivo expansion, persistence, and optimal activity of adoptively transferred pathogen-specific T cells, both CD4+ and CD8+ effector T cells need to be present for efficient protection against viruses,38,39 and CD4+ TH1 cells for protection against fungi.40 The CD154 isolation method favors enrichment of CD4+ T cells because of its predominant expression on activated CD4+ T cells; therefore, it is especially beneficial for MHC class II antigens such as fungal antigens, which primarily induce TH1 immunity. Because CD4+ T cells can show diverse effector functions by inducing different cytokine patterns,40 we also assessed the quality of the C albicans- and A fumigatus–specific CD4+ T-cell response after CD154+ enrichment. The cytokine profile of the fungus-specific T cells was largely predominated by the TH1 cytokines IFN-γ and GM-CSF (data not shown), indicating that the antifungal T cells may be able to activate and support protective the innate immunity that is instrumental in clearing and controlling fungal infections.41
In contrast to fungal infections, viral infections are primarily controlled by cytotoxic CD8+ T cells, but additional activation of CD4+ T cells is inevitable for sustained virus-specific T-cell immunity.42 The precursor frequency of LMP2-specific cytotoxic CD8+ T cells, which was similar to responses described previously,43 was reduced from 80%-97% to only 10%-20% after CD154-based enrichment and expansion. Despite these low numbers of LMP2-specific cytotoxic CD8+ T cells, the single T-cell lines were equally efficient in lysing virus-infected target cells as isolated LMP2-specific CD8+ cytotoxic T cells. In contrast, multipathogen-specific T-cell lines showed a decreased response toward LMP2 peptide-pulsed targets. Moreover, these T-cell lines were only able to lyse virus-infected target cells to a limited extent. This could be compensated for by the isolated CD8+ T-cell fraction of the multipathogen-specific T-cell culture, indicating that this effect is dependent on the amount of antigen-specific CD8+ T cells. These results are in accordance with the recently published tri-virus-specific cytotoxic T-cell lines generated after stimulation with DCs transduced with Ad5f35-pp65/LMP2 vectors,35 and may have been because LMP2 is only weakly expressed on LCLs.44 Whether the amount of LMP2-specific T cells is sufficient in vivo can only be answered in patient studies.
Immunity to AdV is atypical, because this virus shows a predominance of memory CD4+ T cells in the peripheral blood (supplemental Figure 1),38 and the ratio of the enriched CD4+ and CD8+ T-cell population is therefore comparable to the ratio of the precursor cells. This is probably the case because of MHC class I down-regulation in the target cells after infection with AdV to evade immune recognition and the development of a classic, CD8+-mediated cytotoxic adaptive immune response.45 The expansion of AdV-specific T cells was higher compared with previously reports,5,7 probably due to the fact that the CD154-based method predominantly selects CD4+ T cells. Despite the higher number of AdV-specific CD4+ T-cells, the killing of peptide-pulsed target cells was comparable to the killing by CMV- and EBV-specific T-cell lines, particularly in the multipathogen-specific T-cell cultures, suggesting that killing of virus-infected cells is also mediated by cytolytic CD4+ T cells, as has been shown previously.45
Compared with T-cell lines with single-antigen specificity, the frequency of antigen-specific T cells in the multipathogen approach showed a noticeable decrease, probably because of competition between the different antigens, a known phenomenon in multipathogen-specific cultures.5 Remarkably, the T-cell cultures of donors responding to all antigens never lost specificity for any of the 4 or 5 antigens during expansion. Because the required number of infused T cells for a specific antigen has so far not been defined, it remains unclear whether this decrease in antigen-specific T cells in multipathogen-specific lines influences their efficacy in vivo. Previous data have shown that even a single antigen-specific T cell can repopulate distinct T-cell subsets in vivo, and that the success of adoptive T-cell transfer is not necessarily related to the cell dose.36,46 Current clinically applied protocols for adoptive T-cell transfer of viral infections after HSCT used 5 × 104 to 3.5 × 105 cells/kg of in vitro–expanded cells. This number of cells was effective for the reconstitution of pathogen-specific immunity and safe regarding development of GVHD.42,47 Whether the multipathogen approach demonstrated here generates sufficient antigen-specific T cells can only be answered in patient studies. Recent work using the IFN-γ capture assay demonstrated that 1.2−160 × 103 CMV- or AdV-specific T cells/kg with purity above 10% is sufficient to clear or at least decrease virus replication in the majority of patients without causing GVHD. These cells can be infused directly within 48 hours, rendering ex vivo expansion unnecessary.36 In the present study, CD154 isolation resulted in a purity of only 8%-15%, and we could not ascertain the antigen specificity of the isolated cells because of the low numbers of recovered cells. Nevertheless, by generating a clinical-grade, CD154-based selection method, purity might be augmented and the isolated T cells could possibly be similarly infused directly after selection.
In conclusion, we established a simple expansion protocol using a new strategy based on activation-dependent expression of CD154 that could be readily produced under GMP regulations. Within 14 days, allodepleted CD4+ and CD8+ T-cell lines can be generated with high specificity for the most common posttransplantation pathogens. These T-cell lines show extensive proliferative capacity and may further expand if the T cells are stimulated by their natural antigen in vivo. This selection and/or expansion strategy may form the basis for adoptive immunotherapy trials in HSCT recipients at risk for multiple infectious complications and may be translated to a variety of other antigens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Joachim Morschhäuser and Dr Reno Frei for provision of fungal strains, Dr Patricia Comoli and Dr Inge Jedema for provision of LCLs, and Michaela Dümig for excellent technical assistance.
This study was supported by grants from BayImmuNet (to M.S.T.) and the Swiss National Foundation (grant PBBS P3 123164 to N.K.).
Authorship
Contribution: N.K., C.S., S.L., B.C., and C.B. performed the experiments; N.K. and C.S. analyzed the results; S.K. and H.E. provided vital reagents; N.K. and M.S.T. designed the research; and N.K., C.S., and M.S.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Max S. Topp, Medizinische Klinik und Poliklinik II, Universitaetsklinikum Wuerzburg, Oberduerrbacher Strasse 6, 97080 Wuerzburg, Germany; e-mail: topp_m@klinik.uni-wuerzburg.de.



![Figure 3. Antigen-specific T cells in single- and multipathogen-specific lines specifically proliferate upon antigen contact. Proliferation of T-cell lines specific for AdV hexon, EBV LMP2, A fumigatus p41, and/or C albicans MP65 after restimulation with the peptide pool as determined by [3H]-thymidine incorporation after 72 hours. Medians and range of at least 3 independent experiments performed in triplicate of 3 different donors are shown. Reduction of proliferative potential of multipathogen-specific lines versus single lines was only statistically significant for A fumigatus p41 (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2010-12-322610/4/m_zh89991175130003.jpeg?Expires=1769081003&Signature=F1I3nFIfl84~IySjEcHz1GFUUXOQaGuFX7h-nMWCrG1kD1mWtqjh9JtlePJl4eQ3WADlW2C1eCZ39jBWDPHUr33Outo8jItaGIrjUWRNe0Xwu6oeVLuMBRXkW7Z83uyhWep6c-IYCIJ4tEMXJm9ycXCx9eAcZkL9hJStYdMI45lJZWodMTfAxjt61rCewNS7vDtASyy4~ypiRQkQas2dKmSUwgFqMP~nZ9-oXJy1Nl6neFRpDcAN6JAuazXY0xWJNdtLfn1fsJYIfu-OjlIaWt4E6QjLIv5MSjAijEgNXe~BvDEU7ZJqcsnkJHZIJaVSvqE5MyhMECtX-9xuz-QIPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Single- and multipathogen-specific T-cell lines show nearly abrogated alloreactivity. Alloreactivity was determined 14 days after expansion with 2 different third-party DCs by [3H]-thymidine incorporation 96 hours after stimulation. Proliferative decrease of T-cell lines versus autologous PBMCs statistically significant for all T-cell lines (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2010-12-322610/4/m_zh89991175130006.jpeg?Expires=1769081003&Signature=syxiiWdrMolXmnCyU340c5hn0e2IrN8JMuNLpbmt4xCpX4DD8OY~YskKSq1Y-LoMPJ1~t9t0AqaLrAOSDlVpZByTiETv61j8y7J2t3-Fuc9xi6jVbKTjGw-BTITwwqYUkHkNNrjAAvBhXmXl3HYJCrDwyE3oqcABnYoLjbx9O2Vnhkuz-GThX0~QTnP6r825NqHMZxHWcNi5AoZuaJwvWyIUsDO2Ng~IdLQ0oV9fd-ERqgIqVUPdtoZDch1KWssVWgaHkF3ro41JfXRzunzXkgY4E29L5ay8YroDcSYY9rJRs5uKw5f1MjltD5CbmNNVO606ZDe1HVFdtLYiTfwp8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Antigen-specific T cells in single- and multipathogen-specific lines specifically proliferate upon antigen contact. Proliferation of T-cell lines specific for AdV hexon, EBV LMP2, A fumigatus p41, and/or C albicans MP65 after restimulation with the peptide pool as determined by [3H]-thymidine incorporation after 72 hours. Medians and range of at least 3 independent experiments performed in triplicate of 3 different donors are shown. Reduction of proliferative potential of multipathogen-specific lines versus single lines was only statistically significant for A fumigatus p41 (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2010-12-322610/4/m_zh89991175130003.jpeg?Expires=1769081004&Signature=ktMiKiO3E4yZ-koYG0kFTYxWKiR6tMg8fheyT2eD5CnqzqKEhE7g6-cqRpHmpnn2Vr1sTjh2Yb2Jreuj3351bigbsJTaleLdL3CYconxXMYxAHNpHLROYrXVq7RxxCrSEeElKDSzBzCwF7GPKhWtgqL9zdN1jBkS9rjeewtegg5OgeWVhQ5Sb1-FuZbp~sSyE-hlG2Pb0LKKq1McysKbVFzfp6wz2NtW-YpBRM-jwQAtFnulGwSCGIOMV7uXtbllO1UUx~8wMEYxcimmXzIanKxZwK5zyD0ltH2xhzVX93KsMbQ8g3bHFdjByFPC8VxEqUmu3NZ3RufmamYBDANgSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Single- and multipathogen-specific T-cell lines show nearly abrogated alloreactivity. Alloreactivity was determined 14 days after expansion with 2 different third-party DCs by [3H]-thymidine incorporation 96 hours after stimulation. Proliferative decrease of T-cell lines versus autologous PBMCs statistically significant for all T-cell lines (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/4/10.1182_blood-2010-12-322610/4/m_zh89991175130006.jpeg?Expires=1769081004&Signature=hCPIFqtxd3-ucNc~CROhSF5hYCXP6JsdzWIP8yIpwRDFfy16PgBb1pL1d041qeUWOICeirD9pAkLwpp4vaFlCtKe0pwfqLtSZUKSAl2s0Zi7TLO3jdS~d2XIRuESihYOmyBQu3zf~qvXfrmMNJy7UliVHgtGMxmOyQzA7sfYtc1CcO9qOqBZZnJUa1THvN6BSIVZgop8TpmrGVMvQ5DAhIxmKZB8NRfWvEiYjNpUfsUkoIGXvTNJYiSpI3OXRPAwSDQQXoQF1mfvk3ozv8tk4PmpI3E4ggWQLdCNh5XPOo7KQg-Xn4vHeEeTGGenYJxHcatRglX4CL-zxS45phtaJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)