Abstract
Low birth weight (LBW) is associated with increased risk of cardiovascular diseases at adulthood. Nevertheless, the impact of LBW on the endothelium is not clearly established. We investigate whether LBW alters the angiogenic properties of cord blood endothelial colony forming cells (LBW-ECFCs) in 25 preterm neonates compared with 25 term neonates (CT-ECFCs). We observed that LBW decreased the number of colonies formed by ECFCs and delayed the time of appearance of their clonal progeny. LBW dramatically reduced LBW-ECFC capacity to form sprouts and tubes, to migrate and to proliferate in vitro. The angiogenic defect of LBW-ECFCs was confirmed in vivo by their inability to form robust capillary networks in Matrigel plugs injected in nu/nu mice. Gene profile analysis of LBW-ECFCs demonstrated an increased expression of antiangiogenic genes. Among them, thrombospondin 1 (THBS1) was highly expressed at RNA and protein levels in LBW-ECFCs. Silencing THBS1 restored the angiogenic properties of LBW-ECFCs by increasing AKT phosphorylation. The imbalance toward an angiostatic state provide a mechanistic link between LBW and the impaired angiogenic properties of ECFCs and allows the identification of THBS1 as a novel player in LBW-ECFC defect, opening new perspectives for novel deprogramming agents.
Introduction
Epidemiologic and experimental studies have shown that cardiovascular diseases, the leading causes of death in developed countries, have origins during development.1 Low birth weight (LBW) and in particular preterm birth have been associated with long-term hypertension.2,3 The pathways by which cardiovascular diseases are developmentally programmed are multiple, although incompletely understood. Aside the long-term effects of both nephron number reduction4 and altered neuroendocrine control,5 factors that directly affect the vessel structure and function are critical. In particular, LBW is associated with several factors that pave the way toward hypertension, such as altered arterial wall compliance,6 reduced endothelium-dependent vasodilating capacity,7 and incomplete vasculogenesis with reduced microvascular density both in human and animal models.8,9 However, although the endothelium is central in vasculogenic and angiogenic processes, very little information is available on the effect of LBW-associated conditions on the endothelium.
Endothelial dysfunction is defined by loss of regulatory functions related to vascular tone, inflammation, and oxidative stress but also by impaired vasculogenesis and capacity of repair mediated by circulating endothelial progenitor cells (EPCs). First described in blood by Asahara et al,10 EPCs are immature cells which derive from bone marrow, have the capacity to proliferate, migrate, and home to sites of neovascularization and differentiate into mature endothelial cells in situ.11 EPCs play a critical role in vascular repair and new blood vessel formation. Cardiovascular risk factors reduce the availability of EPC, and several works indicated EPC importance in the pathogenesis and prognosis of cardiovascular diseases. Both number and functional activity of EPCs are decreased in patients with stable coronary artery disease,12,13 arterial hypertension,13 hypercholesterolemia,14 or type II diabetes.15 In addition, EPCs are associated with an endothelial dysfunction assessed by altered flow-mediated vasodilatory capacity.16 In coronary artery disease, EPC levels represent the strongest predictor of endothelial dysfunction compared with traditional risk factors.17 Such data emphasize that EPC dysfunction may play a causal role in vascular disease progression.
EPCs represent a small fraction of the blood mononuclear cell population enriched for the stem marker CD34.18 Present at a very low concentration in the adult peripheral blood, their level is increased in cord blood.19 A hierarchical definition of EPCs has been established according to their phenotype and functional properties.18,20 A first population, called colony-forming unit-endothelial cells forms colonies that appear early in culture, display endothelial markers, but do not form vessels in vivo.20,21 A second one, called endothelial colony-forming cells (ECFCs) with high proliferative potential was considered as “late EPC” giving rise to colonies 7 to 21 days after plating. ECFCs display an endothelial phenotype, form clonogenic cell clusters, and have the capacity to incorporate into new blood vessels in immunodeficient mice.18,22 Nevertheless, strictly specific markers allowing the identification of the circulating EPC in blood are still unknown.20,23
There is emerging evidence that deleterious conditions during the fetal life alter EPC function. Along this line, ECFCs from offspring of mothers who had diabetes in pregnancy displayed impaired angiogenic capacity.24 Moreover, microvascular rarefaction that is observed both in humans born with a LBW8 and in a rat model of fetal growth restriction9 could result from decreased vasculogenesis, a function controlled by EPCs. However, little is known on the impact of LBW on the angiogenic properties during the early programming of vascular dysfunction. LBW can result from either intrauterine growth restriction in full-term neonates or from preterm birth. Although both causes of LBW have been epidemiologically associated with developmentally programmed hypertension, the pathophysiology of resulting LBW is completely different. So we only focused our study on the functional properties of ECFCs obtained from cord blood of LBW infants in the context of preterm delivery.
We demonstrate here that the angiogenic properties of ECFCs are impaired in LBW preterm neonates in vitro and in vivo. LBW affects gene expression in ECFCs, leading to an up-regulation of genes with antiangiogenic properties including thrombospondin 1 (THBS1). THBS1 up-regulation and the consequent alteration in AKT phosphorylation markedly reduced ECFC angiogenic properties.
Methods
Expanded information on methods is given in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Patients
Twenty-five term (controls, gestational age [GA] > 37 weeks) and 25 preterm neonates (GA between 27 and 37 weeks, appropriate or small for GA) were included in this study (Table 1). LBW neonates were preterm (GA < 37 weeks) with birth weight < 2700 g (based on national reference curves), neonates < 1500 g were considered as very LBW (VLBW) neonates. LBW was associated with intrauterine fetal growth restriction (IUGR) in 5 of 25 preterm neonates.25 Exclusion criteria were congenital viral infections, major congenital heart malformations, genetic abnormalities, structural brain malformations, and metabolic diseases. This research was approved by the local ethic committee Assistance Publique Hôpitaux de Marseille, and all the parents gave written informed consent for the use of cord blood in accordance with the Declaration of Helsinki.
Circulating EPC quantification
Cord blood circulating EPCs were quantified by flow cytometry using the commercially available Stem Kit (Beckman Coulter).
ECFC culture
ECFCs were isolated from the mononuclear cell fraction (MNC) obtained from umbilical cord blood of controls (CT; > 2700 g) and LBW neonates (between 440 and 2500 g). For this, MNCs from LBW preterm or term neonates were plated at 5 × 106 cells/well in 6-well plates and cultured as described by Ingram et al.18 Colonies of endothelial cells appeared between 5 and 22 days of culture and were identified as well-circumscribed monolayers of cobblestone-appearing cells. ECFCs were enumerated by visual inspection using an inverted microscope (Nikon) under 20×/0.45 magnification. A monolayer containing at least 20 cells with a cobblestone pattern was counted as a colony.
In vitro angiogenesis
Tube formation.
ECFCs were cultured at 20 000 cells/well in 96-well plates coated with 50 μL of growth factor reduced Matrigel (BD Biosciences). Capillary-like structures were counted using ImageJ Version 1.44 software (NCBI). Each experiment was done in triplicate.
Proliferation.
ECFCs were plated in EBM2 media 2% FCS. Proliferation was assayed by 5-bromo-2′-deoxyuridine (BrdU) incorporation using the Cell proliferation assay Kit (Roche Diagnostic).
Spheroid formation.
Spheroids were generated as described by Korff et al.26 Briefly, ECFCs were suspended in culture medium containing 0.2% (weight/volume) carboxymethylcellulose. Spheroids were generated overnight and then embedded into collagen gels. After 24 hours, pictures were captured and cumulative sprout lengths and branch points per spheroid were calculated.
In vivo vasculogenesis
Growth factor reduced Matrigel was filled with a mixture (75:25) of ECFCs and commercially available human umbilical artery smooth muscle cells (SMCs; Promocell) as described by Melero-Martin et al.27 The plugs were subcutaneously injected in the groin lateral areas of nu/nu mice and removed after 1 week, fixed, frozen, and sectioned. Sections were labeled with anti–human CD31 or α-smooth muscle cell actin mAbs, and incubated with goat anti–mouse antibodies respectively labeled with Alexa488 or Alexa546. Colocalization of human CD31 and α-smooth muscle actin staining allowed define blood vessels by fluorescent microscopy magnification 20×/0.45. Blood vessel infiltration in Matrigel plugs was quantified by H&E staining of serial sections and plugs were examined for structures containing or not erythrocytes.
Real time RT-PCR array
Angiogenic gene regulation was performed using the commercially available RT2 Profiler PCR array for human angiogenesis (PAHS-024; SuperArray Bioscience Company) and analyzed as described by the manufacturer. Three independent experiments were performed.
Statistical methods
Demographic data of the preterm and term populations were analyzed with SPSS software using the χ2 test of Spearson for qualitative data and the 2-tailed unpaired t test for quantitative data. Unpaired Student t tests were used for experiments performed in vitro and in mice models using GraphPad, Version 5 software. For all analyses, P < .05 was considered significant.
Results
Reduced colony formation by ECFCs from LBW infants
The relative number of cord blood EPCs from 25 LBW preterm and 25 term neonates (Table 1) was determined by counting CD34+ CD45− events by flow cytometry using the commercially available Stem Kit (Beckman Coulter). No significant difference was observed in the total level of CD34+ CD45− (supplemental Figure 1) between the 2 groups of neonates despite a subtle increase in the blood of VLBW infants (< 1500 g). MNCs isolated from cord bloods (CB-MNCs) were then plated in an endothelial medium. CB-MNCs formed proliferative colonies that contained cells with a cobblestone-like morphology (supplemental Figure 2A) and positively labeled for the endothelial markers CD146 (MUC18), CD31 (PECAM-1), CD144 (VE-Cadherin), and KDR. The cells were weakly positive for the immaturity marker CD34 and negative for CD45, CD14 and CD41, respectively, markers of leukocytes, monocytes, and platelets (supplemental Figure 2B top and bottom patterns). They bound Ulex europaeus lectin (UEA-1 lectin) and stained for DiI-acetylated LDL (supplemental Figure 2C). These data confer to the isolated cells morphologic and phenotypic characteristics of ECFCs.
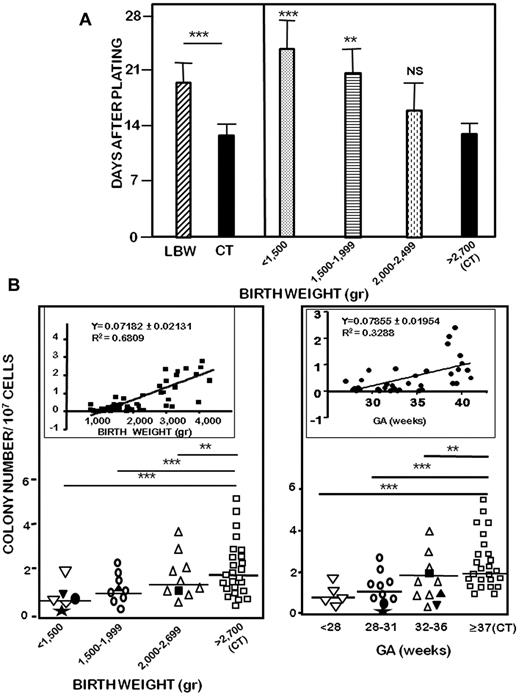
We next investigated the influence of birth weight on the time of appearance and the total number of ECFC colonies obtained after plating. First, LBW-ECFC colonies appeared 17.4 days after MNC seeding, whereas those derived from CT-ECFCs were observed at 12.8 days, a time consistent with the definition of ECFCs (Figure 1A left). There was an inverse relationship between birth weight and the time of appearance of endothelial colonies (Figure 1A right). Second, the quantification of the total number of colonies showed a positive correlation with birth weight (Figure 1B left inset) and with GA (Figure 1B right inset), although a better relationship was observed with birth weight than GA. In case of neonates with IUGR, the numbers of both CD34+ CD45− events and ECFC colonies were comparable with those of infants similar in birth weight rather than GA (supplemental Figure 1; Figure 1B). The reduced capacity of LBW-ECFCs to form colonies was independent of cell death as evidenced by cell viability detected by Alamar blue incorporation and absence of apoptosis detected by annexin-V/7-amino-actinomycin D staining (LBW-ECFCs = 97.1% vs CT-ECFCs = 97.5% viable cells; data not shown). These data show that VLBW (< 1500 g) in preterm neonates greatly reduced the capacity of ECFCs to form colonies and delayed their time of appearance. Taken together, these results indicate that, despite a similar number of ECFCs precursors circulating in cord blood, the capacity of ECFCs to form colonies was altered in LBW preterm infants.
Effect of birth weight on the formation of ECFC colonies. CB-MNCs from 25 LBW preterm or 25 control neonates were seeded onto gelatin-coated wells and observed every 2 days as described in “Methods.” (A) Time-dependent appearance of ECFC colonies after MNC plating of 25 LBW preterm neonates and 25 controls (left), and according to birth weight (right). Data are mean ± SEM of 25 independent experiments. **P < .01. ***P < .001. (B) Numbers of ECFC colonies formed by CB-MNCs according to birth weight (left) or to gestational age (right). (Inset) Spearman correlation coefficient for colony formation in response to birth weight or to gestational age. Filled symbols indicate LBW preterm neonates with IUGR: (★), 680 g, 29 weeks; (●), 930 g, 31 weeks; (▾), 940 g, 32 weeks; (▴), 1700 g, 35 weeks; and (■), 1850 g, 36 weeks. Data represent mean of 25 independent experiments. NS indicates not significant.
Effect of birth weight on the formation of ECFC colonies. CB-MNCs from 25 LBW preterm or 25 control neonates were seeded onto gelatin-coated wells and observed every 2 days as described in “Methods.” (A) Time-dependent appearance of ECFC colonies after MNC plating of 25 LBW preterm neonates and 25 controls (left), and according to birth weight (right). Data are mean ± SEM of 25 independent experiments. **P < .01. ***P < .001. (B) Numbers of ECFC colonies formed by CB-MNCs according to birth weight (left) or to gestational age (right). (Inset) Spearman correlation coefficient for colony formation in response to birth weight or to gestational age. Filled symbols indicate LBW preterm neonates with IUGR: (★), 680 g, 29 weeks; (●), 930 g, 31 weeks; (▾), 940 g, 32 weeks; (▴), 1700 g, 35 weeks; and (■), 1850 g, 36 weeks. Data represent mean of 25 independent experiments. NS indicates not significant.
Impaired angiogenic properties of LBW-ECFCs in vitro
The reduced capacity to form colonies could result from impaired functional properties of ECFCs. To test this hypothesis, we investigated the ability of individual LBW-ECFCs or CT-ECFCs to form progeny. ECFCs were cloned by limiting dilutions and plated at statistically one cell per well. Clone formation by individual LBW-ECFCs was significantly reduced compared with CT-ECFCs (Table 2). In addition, the time of appearance of LBW-ECFC clones was delayed. These data demonstrate impairment in ECFC proliferation.
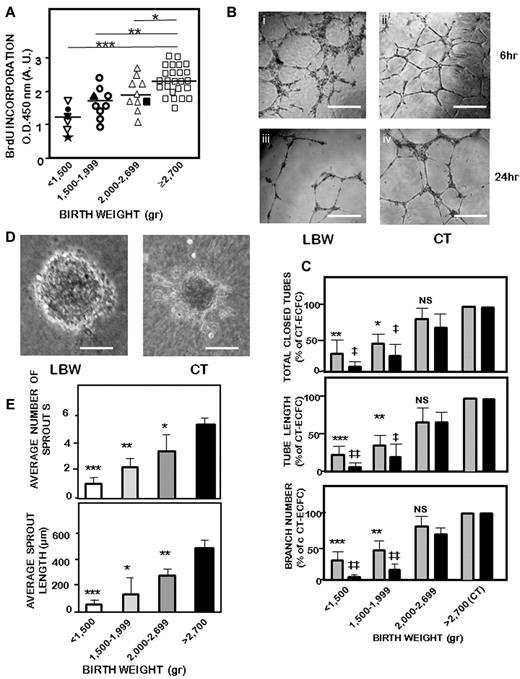
To confirm a proliferative defect of LBW-ECFCs, BrdU incorporation into LBW-ECFCs and CT-ECFCs was determined. Proliferation was significantly decreased in LBW-ECFCs (Figure 2A), the proliferative defect being more marked in ECFCs from infants born with VLBW (< 1500 g).
In vitro angiogenic functions of LBW-ECFCs and CT-ECFCs. ECFCs were used at passage 3 as described in “Methods.” (A) Proliferation assayed by BrdU incorporation in 10 000 ECFCs. Results were expressed in arbitrary units of spectrophotometric measurements. Filled symbols indicate LBW preterm neonates with IUGR: (★), 680 g, 29 weeks; (●), 930 g, 31 weeks; (▾), 940 g, 32 weeks; (▴), 1700 g, 35 weeks; and (■), 2000 g, 36 weeks. Data are mean ± SEM of 25 independent experiments performed in triplicate. *P < .03. **P < .01. ***P < .001. (B) Representative experiment of capillary tube formation by ECFCs from a < 1500-g VLBW neonate and a > 2700-g control neonate. A total of 20 000 ECFCs/well were seeded on top of growth factor-reduced Matrigel. Tube formation was analyzed 6 and 24 hours later on a phase-contrast microscope (original magnification 20×/0.45). Scale bars represent 50 μm. Representative experiment in capillary tube formation by ECFCs from a < 1500-g VLBW neonate at 6 (i) and 24 hr (ii) and a > 2700-g control neonate at the same times (iii-iv). (C) Relative quantification of total tube (top panel), tube length (central panel), and branches (bottom panel) according to birth weight at 6 hours (gray bars) and 24 hours (black bars). Data are mean ± SEM of 25 independent experiments, each performed in triplicate. *P < .05, **P < .01, ***P < .001 vs CT at 6 hours. ‡P < .05, ‡‡P < .02 vs CT at 24 hours. (D) Representative experiment of capillary-like sprout formation from 3D spheroids obtained with ECFCs from < 1500-g extremely LBW neonate (i) and > 2700-g control neonate (original magnification ×20). Scale bars represent 50 μm. Representative experiment of capillary-like sprout formation from 3D spheroids obtained with ECFCs. (E) Quantification of sprout numbers (top panel) and sprout length (bottom panel) formed by ECFCs according to birth weight. Data are mean ± SEM of 10 independent experiments performed in triplicate. ( ), 6 hours: *P < .06, **P < .01, ***P < .001. (
), 6 hours: *P < .06, **P < .01, ***P < .001. ( ), 24 hours: ‡P < .001, ‡‡P < .01. NS indicates not significant.
), 24 hours: ‡P < .001, ‡‡P < .01. NS indicates not significant.
In vitro angiogenic functions of LBW-ECFCs and CT-ECFCs. ECFCs were used at passage 3 as described in “Methods.” (A) Proliferation assayed by BrdU incorporation in 10 000 ECFCs. Results were expressed in arbitrary units of spectrophotometric measurements. Filled symbols indicate LBW preterm neonates with IUGR: (★), 680 g, 29 weeks; (●), 930 g, 31 weeks; (▾), 940 g, 32 weeks; (▴), 1700 g, 35 weeks; and (■), 2000 g, 36 weeks. Data are mean ± SEM of 25 independent experiments performed in triplicate. *P < .03. **P < .01. ***P < .001. (B) Representative experiment of capillary tube formation by ECFCs from a < 1500-g VLBW neonate and a > 2700-g control neonate. A total of 20 000 ECFCs/well were seeded on top of growth factor-reduced Matrigel. Tube formation was analyzed 6 and 24 hours later on a phase-contrast microscope (original magnification 20×/0.45). Scale bars represent 50 μm. Representative experiment in capillary tube formation by ECFCs from a < 1500-g VLBW neonate at 6 (i) and 24 hr (ii) and a > 2700-g control neonate at the same times (iii-iv). (C) Relative quantification of total tube (top panel), tube length (central panel), and branches (bottom panel) according to birth weight at 6 hours (gray bars) and 24 hours (black bars). Data are mean ± SEM of 25 independent experiments, each performed in triplicate. *P < .05, **P < .01, ***P < .001 vs CT at 6 hours. ‡P < .05, ‡‡P < .02 vs CT at 24 hours. (D) Representative experiment of capillary-like sprout formation from 3D spheroids obtained with ECFCs from < 1500-g extremely LBW neonate (i) and > 2700-g control neonate (original magnification ×20). Scale bars represent 50 μm. Representative experiment of capillary-like sprout formation from 3D spheroids obtained with ECFCs. (E) Quantification of sprout numbers (top panel) and sprout length (bottom panel) formed by ECFCs according to birth weight. Data are mean ± SEM of 10 independent experiments performed in triplicate. ( ), 6 hours: *P < .06, **P < .01, ***P < .001. (
), 6 hours: *P < .06, **P < .01, ***P < .001. ( ), 24 hours: ‡P < .001, ‡‡P < .01. NS indicates not significant.
), 24 hours: ‡P < .001, ‡‡P < .01. NS indicates not significant.
In addition to proliferation, angiogenesis requires sprouting, migration, and tube formation. We used 3 in vitro assays to investigate the effect of LBW on ECFC angiogenic potential. First, capillary-like structures on Matrigel formed by LBW-ECFCs were greatly altered 6 hours and 24 hours after plating (Figure 2Bi-ii vs 2Biii-iv). Indeed, they form unclosed, short capillary-like structures (Figure 2Bi) compared with the capillary network promoted by CT-ECFCs (Figure 2Biii) with a significant reduction in the number of total closed tubes, tube length, and branches (Figure 2C). At 24 hours, the alteration of tube formation remained significantly more marked with LBW-ECFCs than that observed with CT-ECFCs, especially in case of VLBW. Second, sprout formation in a 3D-spheroid model was reduced (Figure 2D). Spheroids formed by LBW-ECFCs showed a round shape with very short sprouts (Figure 2Di) compared with those formed by CT-ECFCs (Figure 2Dii). The total number of sprouts and the cumulative length were significantly reduced with LBW-ECFCs (Figure 2E). In an attempt to determine the angiogenic potential of ECFCs from preterm infants with LBW because of IUGR, we compared their capacity to form capillary structures and sprouts with that of ECFCs of neonates with LBW because of only preterm delivery. Capillary-like structures (supplemental Figure 3A left), from LBW-ECFCs associated with IUGR were reduced compared with LBW-ECFCs from preterm neonates with a similar a gestational age (supplemental Figure 3A right) and were identical to those of preterm infants born with a similar weight (supplemental Figure 3A center). Quantification of capillary formation indicated a decrease in total closed tubes, length, and branches by LBW-ECFCs associated with IUGR preterm neonates compared with those obtained with ECFCs from infants similar in gestational age. They were found comparable to LBW-ECFCs from preterm neonates with a similar weight (supplemental Figure 3B). Likewise, sprout formation (supplemental Figure 4A) and the total number of sprouts and sprout length obtained with ECFCs from preterm neonates with IUGR (supplemental Figure 4B) matched more those observed with preterm neonates similar in weight than in gestational age. Third, migration of LBW-ECFCs in a scratched wound assay was also decreased. Whereas CT-ECFCs refilled the empty space 6 hours after having performed a wound, LBW-ECFCs remained less capable of refilling the gap (supplemental Figure 5A left). The defect in the repair was significantly more pronounced in ECFCs from VLBW neonates (supplemental Figure 5A right). All these findings indicate that LBW impairs the in vitro angiogenic properties of ECFCs. As the strongest reductions of angiogenic properties were observed in preterm infants born with VLBW, all the subsequent experiments were performed with ECFCs from preterm infants born with a < 1500-g birth weight.
Impaired capacity of LBW-ECFCs to form blood vessels in vivo
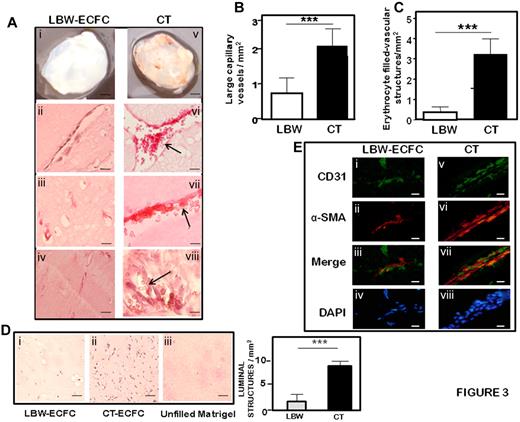
To investigate the capacity of ECFCs to form functional capillary networks in vivo, 1 × 106 LBW-ECFCs or CT-ECFCs were mixed with commercially available human umbilical artery SMC at a ratio of 75:25, included in Matrigel and subcutaneously implanted into nude mice for one week. After harvesting Matrigel implants, macroscopic views revealed a net coloration of the plugs containing CT-ECFCs compared with the implants with LBW-ECFCs (Figure 3Ai vs v). Vessel formation was identified both by histolabeling of the vessel wall and by immunofluorescent staining of vascular markers. H&E staining of 3 different plugs revealed striking differences in the vessels formed by LBW-ECFCs and CT-ECFCs. First, in LBW-ECFC-filled plugs, the vessels appeared thinner than those obtained with CT-ECFCs (Figure 3Aii vs vi) and the number of large capillary structures was significantly reduced (Figure 3B). Second, in the vessels formed by LBW-ECFCs, erythrocytes were hardly found, whereas they filled the vessels formed by CT-ECFCs (Figure 3Aii-iv vs vi-viii) and the difference in the number of vessels containing erythrocytes was highly significant (Figure 3C). Third, luminal microvessels were reduced in LBW-ECFC implants (Figure 3Di vs ii). The vessels stained positive for human endothelial and human umbilical artery SMC with, respectively, human CD31 (Figure 3Ei,v) and α-human smooth muscle actin (Figure 3Eii,vi) in proximity or adjacent to the endothelial cells (Figure 3Eiii,vii), indicating that the human-implanted ECFCs did form functional capillary structures in the plug. As control, implants of Matrigel alone were devoid of vessels (Figure 3Diii). Taken together, these data demonstrate that ECFCs from LBW preterm infants exhibit a critical impairment of their angiogenic potential in vivo.
Formation of vascular networks in vivo with from LBW or CT-ECFCs neonates. A mixture of ECFCs and SMC (ratio 75:25) was resuspended in growth factor-reduced Matrigel and implanted on the groin area of 5-week nu/nu mice. Implants were harvested after 7 days and stained with hematoxylin and eosin or labeled by fluorescent staining as described in “Methods.” (A) Representative serial sections of Matrigel plugs filled with LBW-ECFCs (left panel) or CT-ECFCs (right panel): (i,v) Matrigel plugs recovered from nu/ν mice. H&E staining showing vessel-like structures in plugs filled with: (ii-iv) LBW-ECFCs and (vi-vii) NBW-ECFCs. Arrows indicate the presence of erythrocytes. (B) Quantification of large microvessel density per square millimiter. (C) Counts of erythrocyte-filled vessels per square millimiter. Results represent mean ± SEM from 9 fields in 2 separate sections. ***P < .001. (D) Representative photomicrographs of luminal microvessels in Matrigel plugs filled with: (i) LBW-ECFCs, (ii) CT-ECFCs, and (iii) unfilled Matrigel plug (left). Quantification of luminal structures per square millimiter. Results represent mean ± SEM from 9 fields in 3 separate sections. ***P < .005. (E) Immunofluorescent staining of: endothelial cells (i,v); with anti–human CD31 (green represents SMC) (ii,vi); with anti–human α-smooth muscle actin (red); merged image of CD31 and smooth muscle actin immunostaining (iii,vii); and 4,6-diamidino-2-phenylindole-stained nuclei (iv,viii; blue). Images were representative of implants into 2 mice (original magnification ×40). Scale bars represent 20 μm.
Formation of vascular networks in vivo with from LBW or CT-ECFCs neonates. A mixture of ECFCs and SMC (ratio 75:25) was resuspended in growth factor-reduced Matrigel and implanted on the groin area of 5-week nu/nu mice. Implants were harvested after 7 days and stained with hematoxylin and eosin or labeled by fluorescent staining as described in “Methods.” (A) Representative serial sections of Matrigel plugs filled with LBW-ECFCs (left panel) or CT-ECFCs (right panel): (i,v) Matrigel plugs recovered from nu/ν mice. H&E staining showing vessel-like structures in plugs filled with: (ii-iv) LBW-ECFCs and (vi-vii) NBW-ECFCs. Arrows indicate the presence of erythrocytes. (B) Quantification of large microvessel density per square millimiter. (C) Counts of erythrocyte-filled vessels per square millimiter. Results represent mean ± SEM from 9 fields in 2 separate sections. ***P < .001. (D) Representative photomicrographs of luminal microvessels in Matrigel plugs filled with: (i) LBW-ECFCs, (ii) CT-ECFCs, and (iii) unfilled Matrigel plug (left). Quantification of luminal structures per square millimiter. Results represent mean ± SEM from 9 fields in 3 separate sections. ***P < .005. (E) Immunofluorescent staining of: endothelial cells (i,v); with anti–human CD31 (green represents SMC) (ii,vi); with anti–human α-smooth muscle actin (red); merged image of CD31 and smooth muscle actin immunostaining (iii,vii); and 4,6-diamidino-2-phenylindole-stained nuclei (iv,viii; blue). Images were representative of implants into 2 mice (original magnification ×40). Scale bars represent 20 μm.
Altered expression of genes regulating angiogenesis in LBW ECFCs
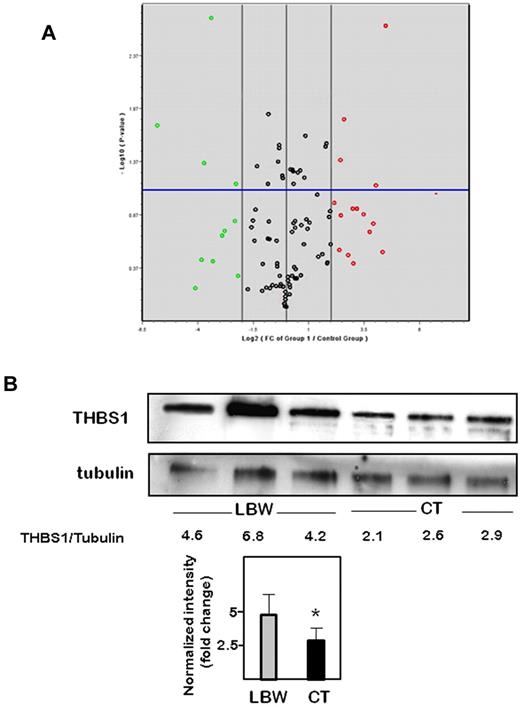
To identify target genes whose expression was modulated in pre-term LBW-ECFCs, we profiled genes essential to angiogenesis using a commercial angiogenesis RT2 profiler PCR array. Among the 84 genes tested, 15 were significantly up-regulated and 11 were down-regulated by more than 2-fold in LBW-ECFCs compared with CT-ECFCs (Figure 4A). Interestingly, there was an up-regulated expression of genes that encoded antiangiogenic factors, such as thrombospondin 1 (THBS1) and 2 (THBS2), tumstatin (COL18A1), brain angiogenesis inhibitor 1 (BAI1), platelet factor 4 (PF4), and angiopoietin 1 (ANGPT1). We observed a down-regulation of genes coding for: (1) angiogenic molecules, such as epidermal growth factor (EGF), neuropilin1 (NRP1), fibroblast growth factor (FGF), and Plexin domain-containing 1(PLXDC1); (2) molecules regulating antiangiogenic factors, such as the inhibitor of DNA 1 (Id1); and (3) chemokines involved in the paracrine activity of ECFCs, such as leukocyte cell-derived chemotaxin 1 (LECT1; supplemental Table 1). These data evidenced an angiostatic profile in ECFC-LBW. Independent quantitative PCR analysis using primer sets different from those used in the array confirmed the up-regulation of THBS1, PF4, and COL18A1 (supplemental Table 2; supplemental Figure 6). Because THBS-1, known as a potent antiangiogenic molecule, showed the highest up-regulation among the genes modulated by LBW, we focused our interest on this protein. Increased THBS1 protein expression was observed in all the 3 LBW-ECFC samples (Figure 4B), confirming the PCR data. Relative quantification indicated that THBS1 levels were significantly higher in LBW-ECFCs than in CT-ECFCs.
Changes in gene expression in LBW-ECFCs. PCR arrays for angiogenesis genes were performed as described in “Methods.” Data represent mean changes of experiments using 3 independent < 1500-g LBW-ECFCs and > 2700-g CT-ECFCs. (A) Volcano plot of angiogenesis PCR array. The x-axis indicates the fold change in Log2 scale; and the y-axis, P value in −Log10 P scale. Using a 2-fold change and a P value of .05 as the cutoff threshold, 11 genes from the left region and 15 from the right region were selected. Red represents high level of expression; green, low level of expression; and black, no change. (B) Representative experiments of THBS1 Western blot in 3 independent < 1500-g LBW-ECFCs or > 2700-g CT-ECFCs. Cell lysate (40 μg) was resolved on 4% to 12% SDS-PAGE gradient under reducing conditions. The blots were probed with anti-THBS1 monoclonal antibody or anti–β-tubulin as loading control. Numbers represent a relative quantification performed by determining the ratio THBS1/tubulin intensities. Bars represent mean ± SEM of the relative intensities. *P < .05.
Changes in gene expression in LBW-ECFCs. PCR arrays for angiogenesis genes were performed as described in “Methods.” Data represent mean changes of experiments using 3 independent < 1500-g LBW-ECFCs and > 2700-g CT-ECFCs. (A) Volcano plot of angiogenesis PCR array. The x-axis indicates the fold change in Log2 scale; and the y-axis, P value in −Log10 P scale. Using a 2-fold change and a P value of .05 as the cutoff threshold, 11 genes from the left region and 15 from the right region were selected. Red represents high level of expression; green, low level of expression; and black, no change. (B) Representative experiments of THBS1 Western blot in 3 independent < 1500-g LBW-ECFCs or > 2700-g CT-ECFCs. Cell lysate (40 μg) was resolved on 4% to 12% SDS-PAGE gradient under reducing conditions. The blots were probed with anti-THBS1 monoclonal antibody or anti–β-tubulin as loading control. Numbers represent a relative quantification performed by determining the ratio THBS1/tubulin intensities. Bars represent mean ± SEM of the relative intensities. *P < .05.
Involvement of THBS1 in the impaired angiogenesis of LBW-ECFCs
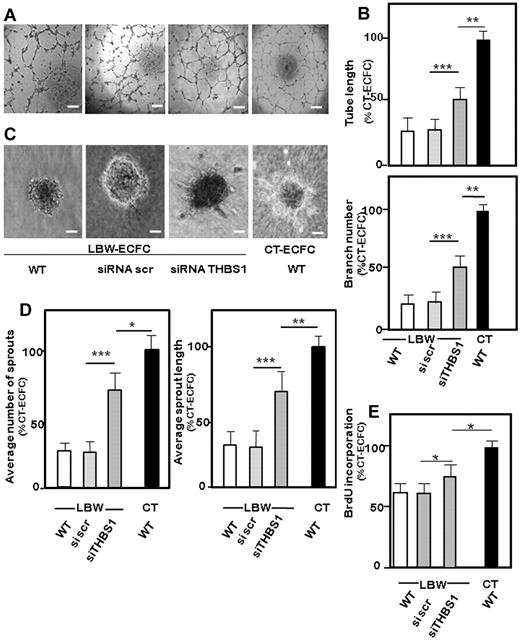
To investigate the contribution of THBS1, we analyzed the effect of THBS1 silencing on the angiogenic properties of LBW-ECFCs. Validated siRNA (supplemental Table 2) caused a significant suppression of THBS1 protein expression after 48 hours (supplemental Figure 6). Compared with silencing with scrambled siRNA, specific knocking down of THBS1 in LBW-ECFCs restored tube morphology (Figure 5A), leading to a significant increase in the formation of total capillary-like tubes in Matrigel (Figure 5B). It also favored the formation of sprouts (Figure 5C) and increased sprout length (Figure 5D); nevertheless, significant differences were always observed between the numbers of capillary structures and angiogenic sprouts formed by THBS1 knocked down LBW-ECFCs and CT-ECFCs. In addition, THBS1 silencing partially increased both proliferation (Figure 5E) and migration (supplemental Figure 5B). Taken together, the data indicated that knocking down THBS1 gene partially restored the in vitro angiogenic properties of LBW-ECFCs.
Involvement of THBS1 in the impaired angiogenic function of LBW-ECFCs. LBW-ECFCs (< 1500 g) were transiently transfected with scrambled siRNA or siRNA specific for THBS1, and cells were recovered 48 hours later for function testing. Representative experiments: (A) Capillary tube formation (original magnification 4×/0.13). Scale bars represent 200 μm. (C) Sprout formation from 3D spheroids (original magnification ×20). Scale bars represent 50 μm by LBW-ECFCs transfected with scrambled siRNA (scr), siRNA THBS1, or from wt-CT-ECFCs. (B) Quantification of tube length and branches by silenced ECFCs. (D) Total number of sprouts by spheroids (left panel) and total length of sprouts (right panel). (E) Proliferation of THBS1-silenced ECFCs: 48 hours after transfection, 10 000 LBW-ECFCs/well were plated, and BrdU incorporation was assayed. Data are mean ± SEM of 3 independent experiments performed in triplicate. *P < .05. **P < .01. ***P < .001.
Involvement of THBS1 in the impaired angiogenic function of LBW-ECFCs. LBW-ECFCs (< 1500 g) were transiently transfected with scrambled siRNA or siRNA specific for THBS1, and cells were recovered 48 hours later for function testing. Representative experiments: (A) Capillary tube formation (original magnification 4×/0.13). Scale bars represent 200 μm. (C) Sprout formation from 3D spheroids (original magnification ×20). Scale bars represent 50 μm by LBW-ECFCs transfected with scrambled siRNA (scr), siRNA THBS1, or from wt-CT-ECFCs. (B) Quantification of tube length and branches by silenced ECFCs. (D) Total number of sprouts by spheroids (left panel) and total length of sprouts (right panel). (E) Proliferation of THBS1-silenced ECFCs: 48 hours after transfection, 10 000 LBW-ECFCs/well were plated, and BrdU incorporation was assayed. Data are mean ± SEM of 3 independent experiments performed in triplicate. *P < .05. **P < .01. ***P < .001.
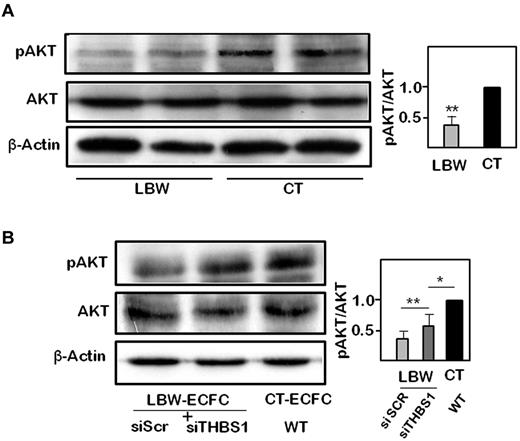
AKT is a central signaling kinase in regulating cell survival, proliferation, and angiogenesis, and its activity is counteracted by THBS1.28 AKT phosphorylation on Ser473 was significantly reduced in LBW-ECFCs compared with CT-ECFCs (Figure 6A). To ascertain the role of THBS1 in the antiangiogenic potential of LBW-ECFCs, we examined changes in AKT phosphorylation after THBS1 silencing. Knocking down of THBS1 in LBW-ECFCs partially restored AKT phosphorylation (Figure 6B). These data indicated that THBS1 controlled the activation of its downstream target AKT in LBW-ECFCs.
Modulation of AKT phosphorylation by thrombospondin 1 in LBW-ECFCs. pAKT and total AKT protein were determined by immunoblot as described in “Methods.” (A) Representative experiment of pAKT and total AKT protein expression in 2 independent < 1500-g LBW-ECFCs and > 2700-g CT-ECFCs. A total of 40 μg of cell lysate was loaded on a 12% acrylamide gel. After transfer, membranes were probed with anti-pAKT, stripped and blotted with antitotal AKT, stripped again, and probed with β-tubulin for charge control. (B) Representative immunoblots of pAKT and total AKT performed on cell lysates (40 μg) from LBW-ECFCs transfected with scr or THBS1 siRNAs. (Inset) Quantification of band intensity of 3 independent immunoblots. Data are mean ± SEM. *P < .05. **P < .01.
Modulation of AKT phosphorylation by thrombospondin 1 in LBW-ECFCs. pAKT and total AKT protein were determined by immunoblot as described in “Methods.” (A) Representative experiment of pAKT and total AKT protein expression in 2 independent < 1500-g LBW-ECFCs and > 2700-g CT-ECFCs. A total of 40 μg of cell lysate was loaded on a 12% acrylamide gel. After transfer, membranes were probed with anti-pAKT, stripped and blotted with antitotal AKT, stripped again, and probed with β-tubulin for charge control. (B) Representative immunoblots of pAKT and total AKT performed on cell lysates (40 μg) from LBW-ECFCs transfected with scr or THBS1 siRNAs. (Inset) Quantification of band intensity of 3 independent immunoblots. Data are mean ± SEM. *P < .05. **P < .01.
Discussion
Epidemiologic studies have shown that antenatal or perinatal adverse events predispose to cardiovascular diseases at adulthood,1 but the underlying mechanisms are not fully understood. This is the first demonstration that a direct relationship exists between birth weight and the functional properties of ECFCs. LBW in preterm neonates impaired cord blood ECFCs angiogenic potential in vitro and greatly reduced their vasculogenic properties in vivo. A mechanistic link between LBW and altered angiogenesis was provided by gene profiling that indicated a shift toward increased expressions of genes with antiangiogenic functions. Among them, THBS1 was up-regulated and contributed to impaired ECFC angiogenic properties through a pathway mediated by AKT, a downstream kinase involved in cell survival.
The novelty of the present study is to report that LBW is another antenatal factor that impairs ECFCs function. Consistently, we observed a positive correlation between birth weight and the number of endothelial colonies in one part and the inverse relationship between birth weight and the delay of colony appearance on the other part. Moreover, the reduced capacity of LBW-ECFCs to give rise to a clonogenic progeny emphasized an intrinsic functional defect of ECFCs because clone formation is governed by the proliferation of an individual cell. The direct demonstration of LBW-ECFC angiogenic impairment was evidenced by in vitro data showing a reduction in sprouts and branches number, tube length, migration, and proliferation in relation with birth weight. Although gestational age represents one of the antenatal factors that affect the number of circulating EPCs,29,30 the functional alteration of LBW-ECFCs did not result from a reduced number of circulating EPCs. Indeed, LBW did not significantly modify the level of cord blood CD34+ CD45− events that were identified as blood circulating ECFC precursors.31,32
None of other maternal or neonatal characteristics in this study predicted the frequency of cord blood ECFCs. Antenatal steroids are administered to the mother to accelerate fetal maturation; thus, antenatal steroids may be considered as a confounding factor in this study. However, corticosteroids have been shown not to affect the in vivo angiogenic potential of cord blood EPCs in a former study,33 and we checked that betamethasone added to culture medium did not show any effect on CT-ECFC angiogenic properties in vitro (data not shown). Moreover, no infants of diabetic mothers (a situation which impaired ECFCs number and function24 ) were included in this study. However, IUGR could be considered as a pejorative factor for the functional activity of ECFCs. Indeed, we showed that ECFCs from preterm infants small for gestational age displayed abnormalities in colony number and in vitro angiogenesis comparable with more immature infants born with the same birth weight. Lastly, no differences were found between infants born from mothers evidencing preeclampsia or not.
In accordance with the in vitro results, the in vivo reduced vasculogenic potency of LBW-ECFCs emphazises the demonstration of the angiogenic defect. LBW-ECFCs formed less robust and functional vascular networks than did CT-ECFCs in vivo. Consistent with the formation of functional anastomoses with the host circulatory system,27 the numbers of erythrocyte-filled vessels were also dramatically reduced in ECFC-LBW implants, indicating a defect in the recruitment of angiogenic cells from the host vasculature. Our results were in agreement with previous observations associating reduced vasculogenesis and vascular density with developmentally programmed hypertension. Indeed, microvascular rarefaction appearing in the neonatal period in rat offspring of protein-deprived dams34 was associated with developmental programming of hypertension and was linked to decreased angiogenesis of aortic rings.9 In addition, the retinal vascular pattern in preterm born women with thoroughly documented blood pressure evidenced a decrease in branching point numbers compared with term born women, which suggests the persistence at adulthood of an abnormal microvascular pattern.8 As in most biologic processes, ECFCs could be influenced by several environmental systemic and local factors. Among many environmental differences, the normal intrauterine environment is markedly hypoxic (partial oxygen pressure = 25 mmHg) compared with the extrauterine, normoxic environment. This major change might be involved in the functional and molecular characteristics of LBW-ECFCs we observed in this work. Indeed, Zhang et al recently demonstrated that chronic hypoxia inhibits the ability of ECFCs to proliferate.35 However, both preterm and control ECFCs are submitted to hypoxia in utero, and we only observed functional defect in LBW-ECFCs when cultured in normoxia. Moreover, partial oxygen pressure is negatively correlated with gestational age.36 In addition, the finding of an altered angiogenic function of LBW-ECFCs may reflect the inappropriate transition to an extrauterine life when it occurs preterm, compared with term birth. Specifically, the maturation of antioxidant enzymes increases in the last weeks of gestation, thus leaving preterm infants highly susceptible to oxidative damage. The demonstration of impaired angiogenesis in vivo indicated an intrinsic defect of LBW-ECFCs to promote neovessel formation in an extrauterine environment, even if it cannot be determined whether such impairment is a physiologic, “transitional” status that may persist over the life course, or an acquired pathologic condition because of perinatal factors.
A novel molecular insight into the cause of LBW-ECFC angiogenic defect was provided by gene expression profiling that evidenced an angiogenesis/angiostatic imbalance in VLBW-ECFCs compared with CT-ECFCs. Changes in the angiogenesis balance encompassed: (1) the up-regulation of molecules with angiostatic properties (thrombospondins,37 CoL18A4,38 and PF439 ), down-regulation of matrix metalloproteases (MMP2 and MMP9) that break down the basement membrane and allow the migration of ECs40 ; and (2) the down-regulation of ubiquitous (VEGF and FGF) or pregnancy specific (PGF) angiogenic factors, molecules associated with tumor proliferation (PLXDC1)41 and cytokines involved in angiogenesis (CXCL1 and CXCL5).42 The pattern of gene expression was consistent with defective vasculogenesis, lack of anastomoses, and vascular rarefaction. Although all these findings do not prove a direct causative relationship between LBW and intrinsic ECFC defect, they greatly suggest that LBW per se contributes to alter ECFCs angiogenic potential.
Among the angiostatic genes, THBS1 plays a key role in the inhibition of angiogenic function of LBW-ECFCs. Indeed, THBS1 protein expression was increased in LBW-ECFCs, confirming the gene profiling data. In addition, specific THBS1 silencing rescued the in vitro angiogenic potential capacity of LBW-ECFCs by increasing the formation of capillary-like tubes and restoring sprout formation in the 3D-angiogenic model of spheroids. The role of THBS1 in angiogenesis is well established. It reduces tumor growth and angiogenesis by inhibiting cell migration, VEGF mobilization, or by directly binding to VEGF.37 The angiostatic effect is also related to a reduction of MMP2 and MMP9.43 Data from PCR array identified correlated changes in the expression of THBS1 and its partners or regulators, such as the transcription factor Id1, which transcriptionally repressed THBS1.44 Id1 reduced expression would favor the up-regulation of THBS1 that in turn reduces MMPs levels. The activity of AKT kinase is negatively controlled by THBS1.37 Reduced phosphorylation of AKT in LBW-ECFCs reflected the impaired angiogenesis linked to the increased expression of THBS1. The recovery of AKT phosphorylation in THBS1–knocked-down LBW-ECFCs emphasized the key role of THBS1 in the control of LBW-ECFC angiogenesis. However, the partial rescue of angiogenesis in response to THBS1 silencing was consistent with an involvement of other molecules in the antiangiogenic state of LBW-ECFCs. As reported in the literature, THBS-1 supports a deleterious impact on cardiovascular diseases.45 THBS1 expression is elevated in the blood vessel wall or surrounding matrix in atherosclerosis,46 tissue ischemia,47 and alters blood pressure.48 Increasing evidence shows that LBW infants develop increased blood pressure at adulthood.3 We can speculate that an increase in THBS1 expression in LBW-ECFCs favors the development of hypertension and increases cardiovascular risks at adulthood.
Besides well-identified key factors that favor the risk of cardiovascular diseases in adult life, epidemiologic studies have evidenced that cardiovascular disease at adulthood is predicted by antenatal events.1 The period that extends from conception to early infancy is critical for alterations that could persist at adulthood.49 Thus, situations that alter EPC functions during fetal and neonatal life, such as hyperglycemia24 or hyperoxia,50 favor the development of cardiovascular diseases. In addition, long-term longitudinal studies in infants and young adults are necessary to determine whether the functional defect could persist along the life span compared with age-matched controls.
In conclusion, the present observation provides evidence for a deleterious role of LBW on the angiogenic properties of ECFCs. To the best of our knowledge, this is the first report to demonstrate a mechanistic link between cord blood ECFCs dysfunction and an up-regulated expression of genes with antiangiogenic properties in LBW preterm neonates. Deciphering the mechanisms that impair ECFCs angiogenic potential will improve our understanding of events impacting the health of adults born with an LBW and will pave the way for novel therapeutic interventions.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. Robert and L. Arnaud (Assistance Publique-Hôpitaux de Marseille) for their help in performing flow cytometric analysis, Ms P. Stellman (Assistance Publique-Hôpitaux de Marseille) for her skillful assistance, and the staffs of the obstetric departments (Conception and Saint Joseph Hospitals, Marseilles), Chiesi Pharmaceuticals, Beckman Coulter, and Stago companies for their support.
This work was supported by the French Ministry of Health (PHRC 2725/5789), Fondation de la Recherche Médicale (grant R08046AA), and Inserm. P.F.V. was funded by a postdoctoral fellowship CAPES/BRAZIL.
Authorship
Contribution: I.L., S.S., and E.T. acquired, analyzed, and interpreted the data; P.F.V. and C.Q. acquired the data; B.G., E.L., G.S., and B.D. performed animal experiments; A.P. and M.M. provided cord blood; A.B. and F.S. made critical revision of the manuscript; U.S. handled funding and supervision and made critical revision of the manuscript; F.D.-G. handled funding and supervision, analyzed the results, and wrote the manuscript; and F.A. designed the research, planned the project, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francine Anfosso, Unité Mixte de Recherche 608, Faculté de Pharmacie, 27 Bd Jean Moulin, 13385 Marseille Cedex 05, France; e-mail: francine.anfosso@univmed.fr.
References
Author notes
I.L., S.S., and E.T. contributed equally to this study.