Abstract
EBV-immortalized B lymphocyte cell lines have been widely banked for studying a variety of diseases, including rare genetic disorders. These cell lines represent an important resource for disease modeling with the induced pluripotent stem cell (iPSC) technology. Here we report the generation of iPSCs from EBV-immortalized B-cell lines derived from multiple inherited disease patients via a nonviral method. The reprogramming method for the EBV cell lines involves a distinct protocol compared with that of patient fibroblasts. The B-cell line–derived iPSCs expressed pluripotency markers, retained the inherited mutation and the parental V(D)J rearrangement profile, and differentiated into all 3 germ layer cell types. There was no integration of the reprogramming-related transgenes or the EBV-associated genes in these iPSCs. The ability to reprogram the widely banked patient B-cell lines will offer an unprecedented opportunity to generate human disease models and provide novel drug therapies.
Introduction
The recent advances in induced pluripotent stem cell (iPSC) research have significantly changed our perspectives on regenerative medicine by providing researchers with a unique tool to derive disease-specific stem cells for study.1 Virus-free generation of human iPSCs from fibroblasts have been reported by the use of various methods, including recombinant proteins,2 piggyBac transposons,3 Epstein-Barr nuclear antigen-1 (EBNA-1)–based episomal plasmids,4 minicircle vectors,5 synthetic RNAs,6 or poly-β amino esters–mediated gene delivery.7
Generation of iPSCs from human blood cells is an attractive option because not only is it much less invasive to obtain peripheral blood, but also because it can take great advantage of the large collections of umbilical cord blood stored in cord blood banks and EBV-immortalized B-cell lines (EBV-B) that have been maintained in a number of institutions such as Coriell Cell Repositories and the UK Biobank. Significant advances in the field include the recent success of episomal plasmids-based reprogramming of human blood cells8,9 as well as a series of viral vector–based blood cell reprogramming.10-16 However, iPSCs have not been derived from EBV-immortalized B-cell lines, which represent an important source of genetic information from patients (and their family members) of various diseases, including rare inherited disorders.17-20 These EBV-B cells can be an excellent resource for disease-specific iPSC generation and banking for a variety of human diseases, especially for those patients with rare diseases whose tissues are no longer available, except as preserved EBV-transformed B cells.
We have demonstrated the iPSC reprogramming potentials of multiple human cell types, including fibroblasts, hepatocytes, mesenchymal stem cells, and blood cells, by using either the conventional retroviral or virus-free methods.10,21-23 By using these approaches, we have successfully reprogrammed fibroblasts obtained from α1-antitrypsin (AAT)–deficient patients. However, most banked patient cells for AAT deficiency (as well as many other diseases) are EBV-transformed B cells, which demonstrates the importance and urgency of developing reprogramming methods for EBV-B cells. We attempted both retroviral and nonviral methods for reprogramming these B-cell lines derived from AAT-deficient patients. The viral method was not successful; however, we were able to reprogram these EBV-B cell lines by using a modified nonviral approach. These EBV-B cell line–derived iPSCs exhibited the characteristics of PSCs, retained the inherited disease-specific donor (G > A) mutation, maintained the rearranged IgG locus, lost the episomal reprogramming genes as well as EBV-related genes, and recapitulated an important disease feature after directed differentiation.
Methods
Patient-derived fibroblasts (4 patients) and EBV-transformed B-cell lines (9 patients) were obtained from Coriell Cell Repositories and cultured according to the Coriell's suggested protocols (http://ccr.coriell.org/Sections/Support/Global/Fibroblast.aspx?PgId = 214, and http://ccr.coriell.org/Sections/Support/Global/Lymphoblastoid.aspx?PgId = 213). The cells were transfected by the use of EBNA-1/OriP–based episomal vectors4 with different nucleofection reagents and conditions (Lonza Walkersville Inc; for details, see supplemental Tables 1 and 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Transfected fibroblasts (Figure 1A) were placed in a standard human embryonic stem cell (hESC) medium10,21 with 0.5mM sodium butyrate23 for 2-3 weeks, and then the visible iPSC colonies were picked and transferred onto mouse embryonic fibroblast (MEF) plates. After transfection, the B-cell lines (Figure 1B) were placed in our modified hESC medium for B-cell reprogramming (DMEM/F12, 20% knockout serum replacement, nonessential amino acid, 20 ng/mL basic fibroblast growth factor, 0.001% β-mercaptoethanol, 0.001% gelatin, 5% protein-free hybridoma medium) with 0.5mM sodium butyrate23 and/or 1μM RepSox24 for 1-2 weeks, followed by replating onto MEF culture plates. At 2-3 weeks later, the iPSC-like colonies were handpicked and propagated by the use of either mouse embryonic fibroblast plates (Millipore) or Matrigel-coated plates (BD).
Reprogramming of EBV-B cells into iPSCs. (A-B) Diagram of the reprogramming process of AAT-deficient patient fibroblasts (A) and EBV-transformed B-cell lines (B). Bright-field images of parental cells before reprogramming and typical colonies right before colony picking are shown. (C-D) Immunofluorescence analysis of pluripotency markers for fibroblast-derived iPSCs (C, iAF3 is shown) and EBV-B cell–derived iPSCs (D, iAB5 is shown). Expression of PSC surface antigens TRA-1-60 and SSEA4 and transcription factors OCT4 and NANOG are observed (magnification, ×200). (E,I) Karyotype analyses for iAF3 and iAB5 lines, respectively. (F-H, J-L) Hematoxylin and eosin staining of teratomas derived from immunodeficient mice injected with either iAF3 (F-H) or iAB5 (J-L) show tissues representing all 3 germ layers (magnification, ×200). Ectoderm (F,J): PE indicates pigmented epithelium; NR, neural rosettes. Mesoderm (G,K): MC indicates mature cartilage; IC, immature cartilage. Endoderm (H,L): RGE indicates respiratory glandular epithelium; IGE, intestinal glandular epithelium.
Reprogramming of EBV-B cells into iPSCs. (A-B) Diagram of the reprogramming process of AAT-deficient patient fibroblasts (A) and EBV-transformed B-cell lines (B). Bright-field images of parental cells before reprogramming and typical colonies right before colony picking are shown. (C-D) Immunofluorescence analysis of pluripotency markers for fibroblast-derived iPSCs (C, iAF3 is shown) and EBV-B cell–derived iPSCs (D, iAB5 is shown). Expression of PSC surface antigens TRA-1-60 and SSEA4 and transcription factors OCT4 and NANOG are observed (magnification, ×200). (E,I) Karyotype analyses for iAF3 and iAB5 lines, respectively. (F-H, J-L) Hematoxylin and eosin staining of teratomas derived from immunodeficient mice injected with either iAF3 (F-H) or iAB5 (J-L) show tissues representing all 3 germ layers (magnification, ×200). Ectoderm (F,J): PE indicates pigmented epithelium; NR, neural rosettes. Mesoderm (G,K): MC indicates mature cartilage; IC, immature cartilage. Endoderm (H,L): RGE indicates respiratory glandular epithelium; IGE, intestinal glandular epithelium.
Characterization of iPSCs was performed by pluripotency marker immunofluorescence and teratoma analysis.10,21,22 Images were taken with the motorized Nikon Ti-E microscope and NIS-Elements software. The presence of EBV genes was assessed by genomic DNA PCR.4,25 Karyotyping of iPSCs was analyzed by G banding.10,21 PCR products (500 bp) of the genomic DNA were used for sequencing (Macrogen) to detect the inherited Z mutations of patient iPSC lines. In addition, V(D)J rearrangement analysis was performed on parental B cells and iPSCs (InVivoScribe Technologies).8 Additional procedures can be found in the supplemental Methods.
Results and discussion
Both fibroblasts and EBV-transformed B-cell lines derived from AAT-deficient patients were reprogrammed via a single transfection of EBNA-1/OriP plasmids encoding reprogramming genes (Figure 1). We used different transfection reagents and methods for these 2 distinct cell types (supplemental Table 1). Transfection efficiencies of ∼ 60% were recorded with B-cell lines and ∼ 97% with fibroblasts (supplemental Table 2). Transfected cells were plated in tissue culture plates immediately after transfection and cultured with either the standard hESC medium (for fibroblasts) or a modified hESC medium (see “Methods”) for supporting repro-gramming of suspension type cells (B cells) for 1-2 weeks (Figure 1A-B).
Adherent iPSC-like colonies appeared in both fibroblast and EBV-B cell reprogramming plates 3-4 weeks after transfection. Generation of EBV-transformed patient B-cell line-derived iPSCs showed a similar reprogramming efficiency to that of iPSC generation from the same disease oriented fibroblasts (∼ 0.002%-0.01%). Extensive characterization was performed on iPSC lines derived from 6 patients (iAF2, iAF3, iAF51, iAF52, iAB5, and iAB9). All iPSC lines expressed pluripotency-associated transcription factors and surface markers, including OCT4, NANOG, and SSEA-4; TRA-1-60 (Figure 1C-D; supplemental Figure 1); and normal karyotypes (Figure 1E,I), and differentiated into all 3 germ layer cell types (Figure 1F-H,J-L, and supplemental Figure 1). By using the same protocol, we also generated 2 iPSC lines (iSB2, iSB3) from sickle cell anemia patient-derived EBV-B cell lines (supplemental Figure 2).
After reprogramming and expansion for more than 10 passages, these iPSC lines lost the reprogramming vectors (Figure 2A) but, as expected, the inherited Z mutation (G > A) in the AAT gene was preserved in the patient iPSC lines (Figure 2B). The inherited mutation does not seem to negatively affect the reprogramming process. These patient-derived iPSCs appear indistinguishable from other human iPSCs (ie iPSCs derived from healthy volunteers or another disease) that do not carry the Z mutation with respect to colony morphology, growth properties, expression of pluripotency-associated markers, and differentiation potentials into all 3 germ layer cell types (Figures 1, 2B). EBV-transformed B-cell–derived iPSC lines also displayed the V(D)J rearrangement profile observed in the parental B-cell lines (Figure 2D).
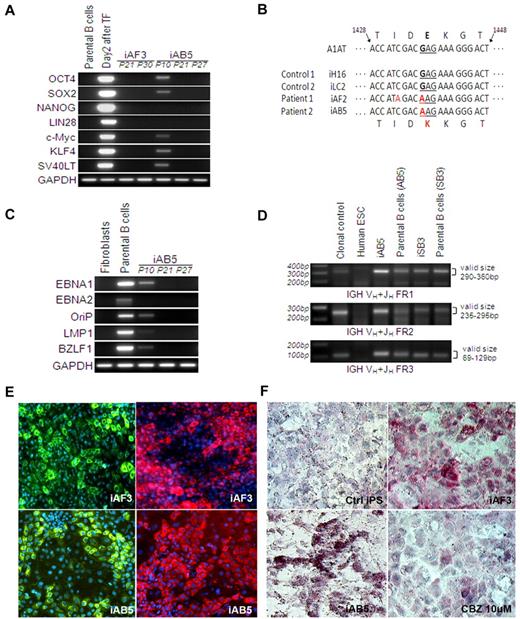
Characterization of the EBV-B cell line–derived iPSCs. (A) Reprogramming genes were not detected in PCR analysis of genomic DNA, indicating generation of integration-free iPSCs. Day 2 transfected cells were included as a positive control, and parental cells were used as a negative control. (B) AAT sequence of human iPSC lines generated from patient fibroblasts and EBV-B cell lines. The inherited Z mutation was preserved after reprogramming of both cell types. Human iPSC lines derived from either healthy volunteers (iH16) or other disease (iLC2, liver cirrhosis), which do not carry the Z mutation, were used as controls. (C) EBV-related genes (EBNA-1, EBNA-2, OriP, LMP-1, and BZLF-1) were analyzed by PCR analysis of genomic DNA obtained from human fibroblasts, parental B cells, and the B-cell line–derived iPSCs. PCR results reveal the absence of EBV DNA in B-cell line–derived iPSCs after passage 20. (D) Three sets of PCR primers were used to detect IGH rearrangements occurring in committed B cells. A clonal control was included as a positive control, and human ESC (H9) was used as a negative control. Both the parental EBV-B cell lines and the cell line–derived iPSC lines showed positive signals of IGH rearrangement. (E-F) Disease modeling potentials. On the basis of our hepatic differentiation protocol, all these patient iPSCs were able to directly differentiate into mature hepatocyte-like cells expressing albumin (green, left) and AAT (red, right); representative images are shown with iAF3 and iAB5 (E). Numerous PASD-positive inclusion bodies (one of the most important AAT deficiency–related liver pathologic features, pink) were detected within mature hepatocytes derived from the AAT patient iPSCs (iAF3, iAB5) but not in the control iPSC (iLC2)-derived hepatocytes (F). The level of PASD-positive inclusion body was decreased with carbamazepine treatment (iAF3, 10μM for 5 days, bottom right).
Characterization of the EBV-B cell line–derived iPSCs. (A) Reprogramming genes were not detected in PCR analysis of genomic DNA, indicating generation of integration-free iPSCs. Day 2 transfected cells were included as a positive control, and parental cells were used as a negative control. (B) AAT sequence of human iPSC lines generated from patient fibroblasts and EBV-B cell lines. The inherited Z mutation was preserved after reprogramming of both cell types. Human iPSC lines derived from either healthy volunteers (iH16) or other disease (iLC2, liver cirrhosis), which do not carry the Z mutation, were used as controls. (C) EBV-related genes (EBNA-1, EBNA-2, OriP, LMP-1, and BZLF-1) were analyzed by PCR analysis of genomic DNA obtained from human fibroblasts, parental B cells, and the B-cell line–derived iPSCs. PCR results reveal the absence of EBV DNA in B-cell line–derived iPSCs after passage 20. (D) Three sets of PCR primers were used to detect IGH rearrangements occurring in committed B cells. A clonal control was included as a positive control, and human ESC (H9) was used as a negative control. Both the parental EBV-B cell lines and the cell line–derived iPSC lines showed positive signals of IGH rearrangement. (E-F) Disease modeling potentials. On the basis of our hepatic differentiation protocol, all these patient iPSCs were able to directly differentiate into mature hepatocyte-like cells expressing albumin (green, left) and AAT (red, right); representative images are shown with iAF3 and iAB5 (E). Numerous PASD-positive inclusion bodies (one of the most important AAT deficiency–related liver pathologic features, pink) were detected within mature hepatocytes derived from the AAT patient iPSCs (iAF3, iAB5) but not in the control iPSC (iLC2)-derived hepatocytes (F). The level of PASD-positive inclusion body was decreased with carbamazepine treatment (iAF3, 10μM for 5 days, bottom right).
EBNA-1 has been reported to be necessary for the establishment of persistent EBV infection and survival of host B cells.26 We tested the presence of EBNA-1 at early (p10) and late passages (p21, p27). EBNA-1 sequence was not detected in the EBV-B cell–derived iPSCs after 20 passages (Figure 2C). The absence of the EBNA-1 sequence along with absence of additional EBV-related genes, OriP, EBNA-2, LMP-1, as well as BZLF-1 (Figure 2C; supplemental Figure 4), might suggest the loss of EBV-associated elements as a result of the reprogramming process.
On the basis of our multistage hepatic differentiation protocol,21,22 all these patient iPSCs could directly differentiate into mature hepatocyte-like cells (Figure 2E). In addition, we observed numerous periodic Acid-Schiff plus diastase digestion (PASD)-positive inclusion bodies (one of the most important AAT deficiency–related liver pathologic features) within hepatocytes derived from the AAT patient iPSCs (Figure 2F). When treated with carbamazepine, which has been reported to decrease mutant AAT formation in nonhuman cell types,27 the hepatocytes derived from patient iPSCs expressed decreased amounts of PASD-positive globules compared with nontreated groups (Figure 2F).
The results of the present study indicate that patient-derived EBV-transformed B-cell lines could be reprogrammed into iPSCs by the use of a virus-free and integration-free method despite the disease-related inherited mutation and that these iPSCs might be useful tools for disease modeling and drug screening/development after directed differentiation into cells of interest.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cyndi Feifei Liu and Jonathan Yen (Johns Hopkins University School of Medicine) for technical assistance and contributions.
This work was supported by Maryland Stem Cell Research Fund grants 106408 and 108563 and in part by National Institutes of Health grant 102681, NYSTEM contract C024406, and the Johns Hopkins Institute for Cell Engineering.
National Institutes of Health
Authorship
Contribution: S.M.C. conducted major experiments (iPSC generation, characterization, and hepatic differentiation) and wrote the manuscript; H.L. helped perform iPSC generation, culture, and differentiation of iPSCs; P.C. aided in iPSC generation and maintenance and in teratoma analysis; Y.K. designed PASD staining and conducted teratoma analysis of iPSCs; L.C. provided critical scientific discussion and wrote the manuscript; J.F. provided scientific discussion and aided in writing the manuscript; S.S. provided scientific discussion and critical reading of the manuscript; Z.Y. generated and characterized iPSCs, analyzed data, and wrote the manuscript; and Y.-Y.J. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhaohui Ye and Yoon-Young Jang, Stem Cell Biology Laboratory, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, 1550 Orleans St, CRB2 Rm 552, Baltimore, MD 21231; e-mail: zye1@jhmi.edu and yjang3@jhmi.edu.