In this issue of Blood, Narla et al show that corticosteroids and lenalidomide used in treating Diamond Blackfan anemia and 5q− syndrome, respectively, have distinct effects on erythropoiesis.1 These data indicate that there may be a role for combining the 2 therapies in treating either disease.
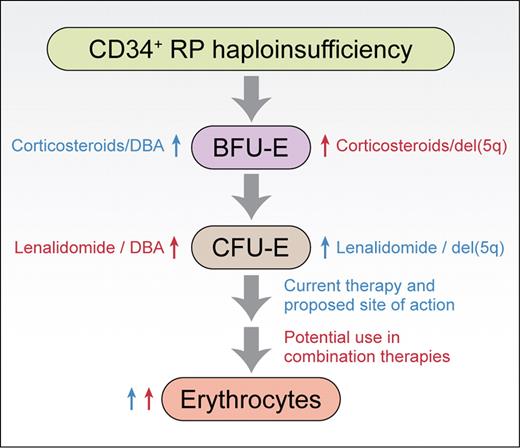
Based on the work of Narla et al,1 the sites of action of corticosteroids and lenalidomide in promoting erythropoiesis from CD34+ cells are shown. Similar sites of action are observed for normal CD34+ cells and CD34+ cells haploinsufficient for ribosomal protein Rps19 or Rps14 (RP haploinsufficiency). Blue labels are given for existing therapies, red labels are provided for potential combination therapies. Professional illustration by Kenneth X. Probst.
Based on the work of Narla et al,1 the sites of action of corticosteroids and lenalidomide in promoting erythropoiesis from CD34+ cells are shown. Similar sites of action are observed for normal CD34+ cells and CD34+ cells haploinsufficient for ribosomal protein Rps19 or Rps14 (RP haploinsufficiency). Blue labels are given for existing therapies, red labels are provided for potential combination therapies. Professional illustration by Kenneth X. Probst.
Diamond Blackfan anemia (DBA) is a congenital bone marrow failure syndrome characterized by a hypoplastic macrocytic anemia.2 RPS19, which encodes a protein of the small ribosomal subunit, was the first DBA gene identified.3 The disease typically shows autosomal dominant inheritance indicating that haploinsufficiency for Rps19 gives rise to DBA. When RPS19 was the only known DBA gene it was unclear if a defect in ribosome synthesis was the underlying cause of DBA or if Rps19 had a more specialized function apart from its role as a structural component of the ribosome that could somehow explain the erythroid specificity of this disease. As more and more DBA genes were identified and shown to encode proteins of either ribosomal subunit, it became increasing clear that DBA was likely a ribosomopathy.4 These studies provided a conceptual framework for the discovery by Ebert et al that the refractory anemia seen in 5q− syndrome was also the consequence of haploinsufficiency for a ribosomal protein, in this case Rps14.5 At the same time, by establishing an independent link between ribosomal protein haploinsufficiency and defective erythropoiesis, the results of Ebert et al put to rest any lingering doubts as to whether or not DBA was a ribosomopathy.
This reciprocal relationship between DBA and del(5q) continues in the current article, where Narla and colleagues investigated the sites of action of corticosteroids and lenalidomide, drugs used to treat the underlying anemias in DBA and 5q− syndrome, respectively.1 These studies were carried out on primary human CD34+ cells induced to differentiate in culture. Their results, supported by a recent publication from Flygare et al using erythroid progenitors from mice fetal livers,6 show that corticosteroids work at the level of BFU-Es, stimulating their self-renewal and amplification. In contrast, lenalidomide was shown by Narla et al to stimulate expansion at the CFU-E stage. These data indicated that the 2 drugs worked by different mechanisms suggesting that effects on erythropoiesis could be enhanced when used in combination, which was indeed demonstrated experimentally. Importantly, in cellular models of DBA and del(5q) where RPS19 and RPS14 expression was reduced using shRNAs, both drugs alone or in combination were shown to increase the production of terminally differentiated erythroid cells from CD34+ progenitors. Together, these results suggest that lenalidomide may have efficacy in treating DBA, corticosteroids may have a role in treating 5q− syndrome, and that combining drugs may be efficacious for both diseases (see figure).
Despite these results, significant concerns exist in terms of translating these observations to the clinic. Most of these concerns relate to the potential use of lenalidomide as a treatment for patients with DBA. The pathophysiology of del(5q) is driven by acquired deletions of segments of the long arm of chromosome 5. These deletions include a common deleted region thought to encompass a minimum of 44 genes including RPS14.7 As a consequence of these deletions, cells acquire a growth advantage allowing the clonal outgrowth of cells unable to efficiently differentiate along the erythroid lineage. While the deletion of RPS14 appears to explain the inability of these cells to differentiate into erythrocytes, it is much more difficult to envision how the deletion of a gene encoding a ribosomal protein could provide a growth advantage to these clones. Thus, presumably 1 or more of the other genes deleted along with RPS14 provides this clonal advantage. More importantly for the present discussion is the mode of action of lenalidomide in treating 5q− syndrome. Lenalidomide has been reported to act by promoting erythropoiesis from the malignant clone and/or by selective apoptosis and elimination of the malignant clone.8 If apoptosis and elimination of the malignant clone is the predominant mode of action of lenalidomide, then using this drug for DBA where each cell harbors the congenital mutation could conceivably induce aplasia. The results presented by Narla and colleagues favor the view that lenalidomide acts by promoting erythropoiesis without an effect on apoptosis, at least in the tissue culture setting where haploinsufficiency is limited to a single ribosomal protein.1 This view is consistent with previous studies that indicate genes other than RPS14 found in the 5q minimally deleted region are responsible for the proapoptotic effects of lenalidomide.9 Consequently, the prospects for the potential use of lenalidomide as a treatment option for DBA look encouraging. Clinical trials will ultimately determine whether this promise will be borne out.
This report by Narla et al shows how studies on DBA and del(5q) continue to inform one another. This reciprocal relationship has allowed for tremendous advances in our understanding of the underlying pathophysiology of both diseases. Moreover, this progress is poised to translate into more effective treatments for these congenital and acquired bone marrow failure syndromes.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■