Whether or not vertebrate blood clotting has a major role in defending against microbial infection has long been a matter of debate. Apart from the mere physical barrier that a fibrin clot presents against external invaders when plugging a wound, is there a strategy specifically targeted against the pathogen?
In this issue of Blood, Loof et al have achieved a major step toward answering that question by definitively showing that the bacterium Streptococcus pyogenes not only provokes coagulation but when it subsequently becomes entrapped in the fibrin clot, the two entities becoming covalently cross-linked by the action of coagulation factor XIIIa.1 Convincing evidence is provided by adroit comparisons of plasma from normal and factor XIII–deficient persons, as well by experiments with wild-type and factor XIII-null mice.
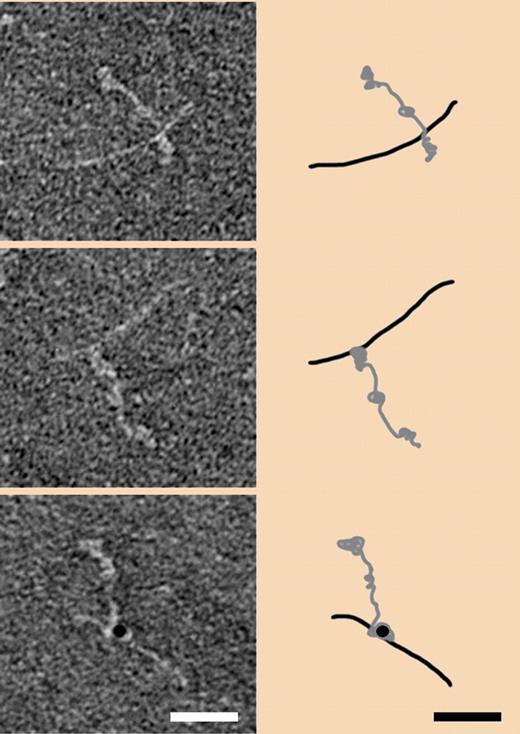
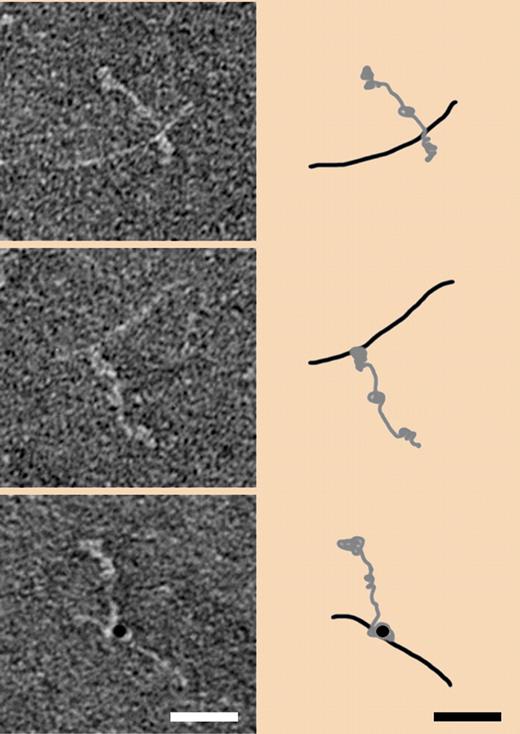
The electron micrographs (left side) are of negatively stained human fibrinogen complexed with the M1 protein of S pyogenes before (top) and after factor XIII–induced cross-linking (middle). Cross-linking detected with gold-labeled antibody is shown in the bottom panel. Corresponding interpretive schematics are shown on the right side. Taken from Figure 3A in Loof et al, page 2589.1
The electron micrographs (left side) are of negatively stained human fibrinogen complexed with the M1 protein of S pyogenes before (top) and after factor XIII–induced cross-linking (middle). Cross-linking detected with gold-labeled antibody is shown in the bottom panel. Corresponding interpretive schematics are shown on the right side. Taken from Figure 3A in Loof et al, page 2589.1
Factor XIIIa is a transglutaminase, an enzyme that catalyzes the formation of covalent bonds between lysine amino groups and suitably exposed glutamine carboxamide groups. The family is ubiquitous in animals, playing roles as divergent as strengthening the epithelial barrier of skin2 and forming a vaginal plug in mammals3 to refashioning the cuticle during insect metamorphosis.4 Auspiciously, transglutaminases have also been found to participate in the independently evolved clotting schemes of insects,5 arthropods,6 and arachnids,7 as well as vertebrates.
In their study, Loof et al show convincingly that the entrapment of S pyogenes in clots is dependent on factor XIII, occurring only in clots formed with thrombin-activated, normal human plasma and not in clots made with plasma from factor XIII-deficient persons.1 Moreover, factor XIII-null mice suffered greater pathologic damage at sites of infection. Remarkably, a commercial preparation of transglutaminase (Fibrogammin P), when added exogenously, also slowed bacterial spreading in infected mice.
The study by Loof an colleagues also employed a gold-label version of an antibody to the ϵ-amino-γ-glutamyl cross-link in conjunction with electron microscopy to pinpoint locations in the fibrin(ogen)-bacterial systems. In particular, they have followed up on their earlier work with the M1 protein of these bacteria, which they had shown was the entity that forms the complex with fibrinogen.8
Given the convincing evidence that factor XIII plays a central role in affixing the M1 protein from S pyogenes to fibrin clots, it needs to be asked, how does it happen? Transglutaminases are notoriously nonspecific with regard to sequences surrounding the ultimately involved lysines and glutamines, and typically the targeted regions are brought into proximity by other forces. For example, in the case of γ-chain cross-linking in vertebrate fibrin, it is the noncovalent polymerization of fibrin units that brings the carboxyl segments of γ-chains near each that allows the rapid formation of the γ-γ cross-links by factor XIIIa. The question arises; does a comparable situation exist in the cross-linking of the S pyogenes M1 protein to the fibrin clot?
Very recently Macheboeuf et al obtained a crystal structure of a complex of the Streptococcal M1 protein with the 86 kDa fragment D of human fibrinogen.9 The interaction between the two was shown to involve a repeat region of the coiled-coils of the M1 protein with the coiled-coil stub of fragment D near its junction with the globular terminal region. The criss-crossed nature of the crystal packing led the authors to propose a fibrinogen-M1 network as the provoking source of neutrophil recruitment.9
A comparable picture is presented in a figure by Luff et al in which they show negatively stained electron micrographs of the M1 protein-fibrinogen complex before and after factor XIIIa cross-linking (see figure). Even with allowance being made for the difference in resolution between the X-ray study and the electron micrographs, the arrangement of the two entities before cross-linking seems the same (top panel). After cross-linking, however, the point where the M1 protein is joined seems significantly nearer the carboxyl terminal of fibrinogen (middle panel), a location where ordinary fibrin cross-linking occurs, as well as where numerous other bacteria and platelets are known to adhere.10 In any case, it seems important to establish the exact locations of cross-links between bacteria and fibrinogen, and future studies will doubtless focus on the point.
Meanwhile, it could be asked, does the resulting cross-linked network favor the host or the invader? In the complicated give-and-take of microbial infections, the advantage of any action to the host or invader can shift with conditions. Certainly the M1 protein of S pyogenes is an important virulence factor that can complex with the host fibrinogen and induce the neutrophil activation that ultimately leads to hazardous vascular leakage and general inflammation.8 It has also been suggested that binding of fibrinogen is primarily to avoid phagocytosis by host cells.11 So to find now that the very same complex leads to the lethal entrapment of the bacterium is to turn the tables.
In an earlier study, this group had shown that entrapment of Escherichia coli and Staphylococcus aureus occurs in plasma clots from normal humans but not in clots from factor XIII–deficient persons.12 It will be of interest to find where factor XIIIa–induced cross-links form in those bacteria that do not have M1 proteins.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■