Abstract
Immunodeficient mice engrafted with human HSCs support multidisciplinary translational experimentation, including the study of human hematopoiesis. Heightened levels of human HSC engraftment are observed in immunodeficient mice expressing mutations in the IL2-receptor common γ chain (IL2rg) gene, including NOD-scid IL2rγnull (NSG) mice. Engraftment of human HSC requires preconditioning of immunodeficient recipients, usually with irradiation. Such preconditioning increases the expression of stem cell factor (SCF), which is critical for HSC engraftment, proliferation, and survival. We hypothesized that transgenic expression of human membrane-bound stem cell factor Tg(hu-mSCF)] would increase levels of human HSC engraftment in nonirradiated NSG mice and eliminate complications associated with irradiation. Surprisingly, detectable levels of human CD45+ cell chimerism were observed after transplantation of cord blood–derived human HSCs into nonirradiated adult as well as newborn NSG mice. However, transgenic expression of human mSCF enabled heightened levels of human hematopoietic cell chimerism in the absence of irradiation. Moreover, nonirradiated NSG-Tg(hu-mSCF) mice engrafted as newborns with human HSCs rejected human skin grafts from a histoincompatible donor, indicating the development of a functional human immune system. These data provide a new immunodeficient mouse model that does not require irradiation preconditioning for human HSC engraftment and immune system development.
Introduction
The development of immunodeficient mouse strains that support engraftment with human hematopoietic cells began with the discovery of the Prkdcscid (scid) mutation on the CB-17 strain background in 19831 followed shortly thereafter with reports of engraftment of human HSCs2 and PBMCs.3 Human HSC engraft in these mice but only at very low levels in part because of heightened innate host immune function, including high levels of NK cell activity.4,5 However, crossing the scid mutation onto the NOD strain decreased NK cell and innate immune function, resulting in increased levels of human HSC engraftment,4,5 yet this engraftment requires preconditioning with irradiation. Even with higher levels of HSC engraftment, NOD mice bearing the scid mutation generated an incomplete human immune system, with minimal development of human T cells.6
A major technologic breakthrough occurred when investigators created genetic stocks of scid, and recombination activating gene 1null (Rag1null) or Rag2null mice that also harbored mutations in the IL-2 receptor common γ chain (IL2rg) gene (abbreviated as IL2rγ).5 IL-2rγ-chain function is required for high affinity ligand binding and signaling through receptors for multiple cytokines, including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21.5 Immunodeficient mice bearing a mutated IL2rγ gene support significantly higher levels of human hematopoietic cell chimerism than all previous immunodeficient stocks studied. After engraftment of human HSCs, development of multiple immune lineages leads to the generation of a functional human immune system. Newborn immunodeficient NOD-scid mice bearing the IL2rγnull targeted mutation (NOD.Cg-Prkdcscid IL2rgtm1Wjl, abbreviated NSG) have higher levels of human cell chimerism than do BALB/c-Rag2null IL2rγnull mice or adult NSG mice.7 Preconditioning of newborn and adults with irradiation is routinely used before human HSC engraftment in all strains of immunodeficient mice bearing targeted mutations in the IL2rγnull gene,5,8 although there is a report of engraftment of human HSC into nonirradiated NSG mice using intrabone marrow injection after nonmyeloablative conditioning.7 Irradiation of mice results in increased expression of stem cell factor (SCF), which enhances hematopoiesis and survival.9
In 1988, mutations in the gene responsible for the severe macrocytic anemia at the W locus in mice were identified in the c-kit (Kit, stem cell factor receptor) proto-oncogene.10 SCF (also known as KitL), the ligand for c-Kit, was subsequently shown to exist in multiple forms, including membrane-associated SCF248, membrane-associated SCF220, and soluble SCF.11-15 SCF is critical for early phases of hematopoiesis, facilitates HSC proliferation and survival, and in vitro treatment of HSC with SCF enhances engraftment in vivo.16 Based on the importance of SCF in HSC development, transgenic expression of human SCF was predicted to enhance the engraftment of human HSCs in immunodeficient mice. A number of mouse strains transgenically expressing various forms of SCF have been generated, including NOD-scid mice expressing 3 cointegrated human genes encoding soluble SCF, IL-3 and GM-CSF.17,18 However, engraftment of human HSCs in these NOD-scid triple transgenic mice resulted in decreased human cell chimerism of the stem cell compartment within the bone marrow while expanding terminal myelopoiesis.17 More recently, these 3 transgenes have been crossed to NSG mice and were found to support regulatory T-cell development after human HSC engraftment and enhanced primary human acute myeloid leukemia engraftment.19,20
Mice transgenically expressing the membrane-bound form of human SCF220 [Tg(hu-mSCF)] were initially generated on the C3H/HeJ strain, and the effect of this transgene on murine hematopoiesis has been described.21,22 However, NSG mice transgenically expressing membrane-bound SCF have not been previously generated to test their ability to support engraftment with human HSCs. We backcrossed the Tg(hu-mSCF) transgene onto the NSG strain to determine whether expression of hu-mSCF would enhance human cell chimerism and overcome the requirement of irradiation conditioning for efficient engraftment. We observed that levels of human cell chimerism in nonirradiated newborn NSG-Tg(hu-mSCF) mice were equivalent to that observed in newborn NSG mice preconditioned with 100 cGy. Moreover, the engrafted human HSCs generated a functional human immune system capable of rejecting histoincompatible human skin grafts. In contrast, radiation preconditioning was necessary for human cell chimerism in both NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/SzJ (NRG) and NRG-Tg(hu-mSCF) mice. These data show that expression of Tg(hu-mSCF) in immunodeficient Prkdcscid mice permits engraftment and differentiation of human HSCs in the absence of preconditioning irradiation and that this effect is dependent on the strain background.
Methods
Mice
NOD.Cg-Prkdcscid IL2rgtm1Wjl/ SzJ (NSG)23 and NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/SzJ (NRG) mice have been described.23,24 Congenic strains of NSG and NRG mice expressing the 220-amino acid isoform of the human membrane-bound stem cell factor (mSCF220) were derived from genetic crosses with hemizygous mSCF220 transgenic mice initially generated on the C3H/HeJ background (C3H/HeJ-Tg(PGK1-KITLG*220)441Daw).21 These transgenic mice express human membrane-bound SCF220 from a cassette, including the human SCF220 cDNA expressed from the human phosphoglycerate kinase (PGK) promoter. The hemizygous C3H/HeJ-Tg(hu-mSCF) transgenic mice were first mated with NOD.Cg-Prkdcscid mice. After backcrossing the transgene to NOD.Cg-Prkdcscid for 10 generations, the NOD.Cg-Prkdcscid-Tg(hu-mSCF) mice were mated with NOD.Cg-Prkdcscid IL2rgtm1Wjl mice. In additional crosses, the hu-mSCF transgene, scid, and IL2rγnull genes were fixed to homozygosity. PCR reactions for typing the scid and IL2rγnull genes have been described.23 The PCR reaction used to identify hu-mSCF220 transgenics (both hu-mSCF220/+ hemizygotes and hu-mSCF220/hu-mSCF220 homozygotes) used primers to amplify a region of the huPGK-1 promoter included in the transgene. The sequences of these primers are as follows: PGKtv-F, 5′-aca ttc tta cgt ccg ttc gc-3′; and PGKtv-R, 5′-act agt gag acg tgc ggc tt-3′.21 The NSG transgenic immunodeficient mice are designated NOD.Cg-Prkdcscid IL2rgtm1Wjl Tg(PGK1-KITLG*220)441Daw/J, abbreviated as NOD-scid IL2rγnull Tg(hu-mSCF220) or NSG-Tg(hu-mSCF) mice. Genetic crosses between NSG-Tg(hu-mSCF) mice and NRG mice were carried out to replace the scid mutation with the wild-type allele and to fix the Rag1null targeted mutation to homozygosity. These NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/SzJ Tg(PGK1-KITLG*220)441 Daw /J mice are abbreviated as NRG-Tg(hu-mSCF) mice. Both the NSG-Tg(hu-mSCF) and NRG-Tg(hu-mSCF) mice are maintained by brother-sister mating of homozygous offspring, respectively.
All animal use was in accordance with the guidelines of the Animal Care and Use Committee of the University of Massachusetts Medical School and The Jackson Laboratory and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996).
Antibodies and flow cytometry
The phenotypes of murine cells were determined as described23 (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For analysis of human hematopoietic engraftment, anti–human mAbs were purchased as FITC, PE, allophycocyanin, peridinin chlorophyll protein, AlexaFluor-700, Pacific Blue, and PECy7 conjugates. This antibody panel accommodated 7-color flow cytometric analysis in the combinations indicated in Table 2 and supplemental Tables 3 through 5. Anti-CD3, -CD4, -CD8, -CD10, -CD14, -CD20, -CD33, -CD34, -CD45, -CD45RA, -CD45RO, -CD64, and -CD71 were purchased from BD Biosciences. Anti–human FoxP3 was purchased from eBioscience. Anti–human CD123 and BDCA2 were purchased from Miltenyi Biotec. Anti–human CD11c was purchased from BioLegend, and anti–human CD235a was purchased from Coulter Immunotech.
Single-cell suspensions of bone marrow, spleen, and thymus were prepared from nonengrafted and human HSC-engrafted mice. Whole blood was collected in heparin. Single-cell suspensions of 1 × 106 cells in a 50-μL volume or 100-μL of whole blood were preincubated with rat anti–mouse FcR11b (clone 2.4G2) to block Fc binding. Cells were prepared for flow cytometry as described.8,23,25 At least 50 000 events were acquired on LSRII or FACSCalibur instruments (BD Biosciences). Data analysis was performed with FlowJo Version 9.4.10 (TreeStar) software.
Radiation sensitivity
Cohorts of NSG and NSG-Tg(hu-mSCF) 90-day-old male and female mice were exposed to various doses of whole body irradiation at a rate of approximately 139 cGy/min from a Shepard Mark I irradiator containing 137CS (J.L. Shepard). The mice were examined daily and killed by carbon dioxide asphyxiation when moribund. Surviving mice were killed at 125 days after irradiation.
Engraftment of mice with human HSCs
Umbilical cord blood was obtained in accordance with the Committee for the Protection of Human Subjects in Research guidelines of the University of Massachusetts Medical School. Umbilical cord blood was provided by the medical staff of the University of Massachusetts Memorial Umbilical Cord Blood Donation Program. Groups of 24- to 72-hour-old (newborn) NSG and NSG-Tg(hu-mSCF) mice were either nonirradiated or were irradiated with 100 cGy.25 Adult NSG and NSG-Tg(hu-mSCF) mice were nonirradiated or irradiated with 240 cGy and transplanted intravenously with human HSCs via the tail vein. Nonirradiated or 400-cGy irradiated newborn NRG mice and NRG-Tg(hu-mSCF) mice were also engrafted with human HSCs. We injected irradiated and nonirradiated mice with CD3 T cell–depleted human umbilical cord blood containing 3 × 104 CD34+ HSCs in a 25- to 50-μL volume via intracardiac injection.8 After 12 weeks, flow cytometric analyses of HSC recipients determined the engraftment of the human immune system.
Skin transplantation protocol
Human skin from panniculectomy operations was obtained in accordance with the Committee for the Protection of Human Subjects in Research guidelines of the University of Massachusetts Medical School. HSC- and non-HSC–engrafted mice treated with anti–mouse Gr-1 monoclonal antibody were transplanted with human split-thickness skin grafts as previously described.26
Hematologic analyses of nonengrafted mice
Blood was collected from the retro-orbital venous plexus of 8- to 10-week-old mice using EDTA-coated capillary tubes (Drummond Scientific). Erythrocytes, leukocytes, and platelets were analyzed using an ADVIA 120 Hematology System (Bayer Corp) as previously described.27
Histology and histochemistry
Tissues were recovered, fixed in 10% neutral buffered formalin, and processed as described for histology and histochemistry.28 Briefly, fixed tissues were embedded in paraffin, and 5-m section were prepared from the blocks.
Sections were stained for H&E and immunostained with monoclonal antibodies specific for human CD45 (Dako Denmark), human CD31 (Dako Denmark), human involucrin (Santa Cruz Biotechnology) and human vimentin (GenWay). Sections were maintained without any medium. Digital light microscopic images were recorded, at room temperature, with a Nikon EclipseE600 microscope (with 10× and 20× Nikon objective lenses), a Diagnostic Instruments Spot RT color camera, and Spot 5.0 Basic software.
Colony-forming cell assay
Single-cell suspensions from the bone marrow of the indicated mice were stained with mAb specific for human CD45, CD34, and CD38. Human CD34+/CD38− and CD34+/CD38+ cell populations were purified using a BD FACSAria cell sorter (BD Biosciences) to a purity level of greater than 95%. The hematopoietic potential of the recovered cell populations was determined in a colony-forming cell assay as described by the manufacturer (StemCell Technologies).
Statistical analyses
Data are presented as the mean plus or minus SD unless other stated. The flow cytometric data on human engraftment are presented as mean plus or minus SD. To compare individual pair-wise groupings, we used 1-way ANOVA with Bonferroni posttests and Kruskal-Wallis test with Dunn posttest for parametric and nonparametric data, respectively. Significant differences were assumed for P values less than .05. Statistical analyses were performed using GraphPad Prism Version 4.0c software.
Results
NSG-Tg(hu-mSCF) mice have normal hematologic profiles and increased susceptibility to irradiation
NSG-Tg(hu-mSCF) mice were generated and bred to homozygosity as described in “Mice.” To determine whether transgenic expression of hu-mSCF altered the hematologic profile of NSG mice, bone marrow flow cytometric analyses were performed comparing hematopoietic cell populations in the bone marrow of NSG and NSG-Tg(hu-mSCF) mice. Small differences were observed in the Gr1+Mac1+ myeloid population and in MHC class I+ cells, but not in other populations (supplemental Table 1).
We next determined the hematologic profile of the blood of NSG and NSG-Tg(hu-mSCF) mice. No significant difference was seen in white blood cell or mature red blood cell numbers or mean cell volumes (supplemental Table 2). Reticulocyte percentages and numbers were significantly higher in NSG-Tg(hu-mSCF) mice, suggesting that an anemia was being compensated for by increased erythrocyte production. Platelet numbers overall appeared lower in NSG-Tg(hu-mSCF) mice, but this was not significant because of large variances. All other populations measured were not significantly different from nontransgenic NSG control mice.
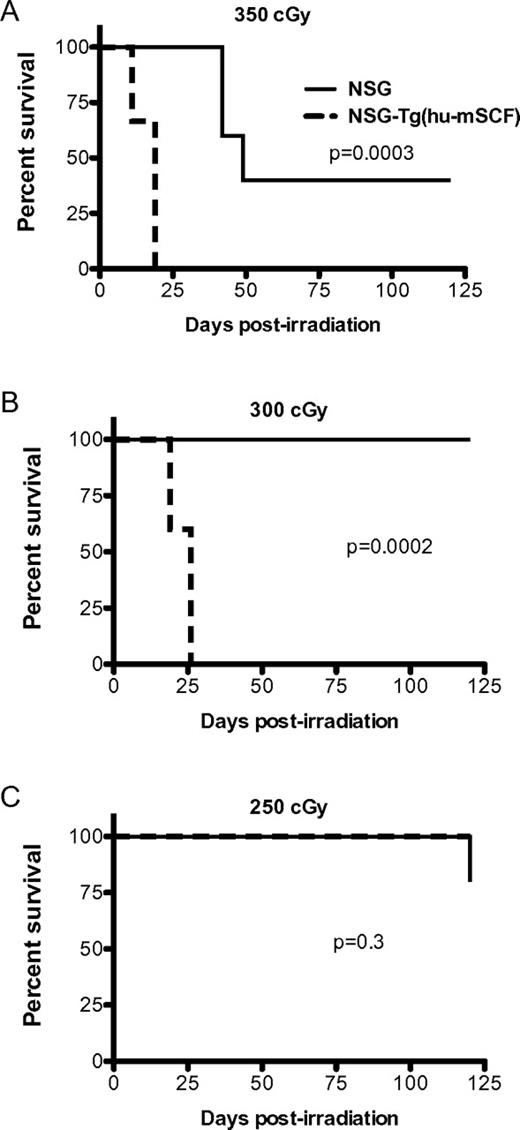
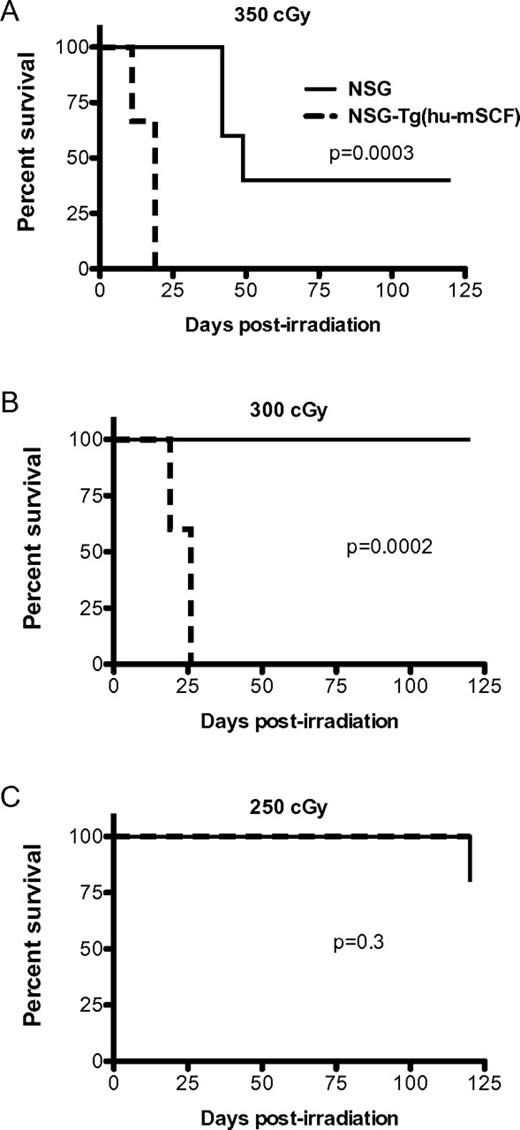
Previous studies have demonstrated that transgenic expression of human membrane-bound SCF in mice antagonizes the normal function of murine SCF and results in the development of abnormalities similar to that observed in mice lacking SCF signaling.9,22 Given the importance of SCF in enhancing survival after irradiation, we hypothesized that the expression of human SCF would increase the radiosensitivity of NSG mice. To test this, we irradiated NSG and NSG-Tg(hu-mSCF) with 350, 300, or 250 cGy and followed the mice for survival (Figure 1). The NSG-Tg(hu-mSCF) mice were significantly more sensitive to irradiation with high levels of mortality at the 350- and 300-cGy doses.
Radiosensitivity of NSG and NSG-Tg(hu-mSCF). NSG and NSG-Tg(hu-mSCF) mice were irradiated with 350 cGy (A), 300 cGy (B), or 250 cGy (C). Mice (5 mice per group) were then monitored for survival.
Radiosensitivity of NSG and NSG-Tg(hu-mSCF). NSG and NSG-Tg(hu-mSCF) mice were irradiated with 350 cGy (A), 300 cGy (B), or 250 cGy (C). Mice (5 mice per group) were then monitored for survival.
Human HSC-engrafted adult NSG and NSG-Tg(hu-mSCF) mice support human hematopoietic cell chimerism in the absence of irradiation
We next determined whether transgenic expression of hu-mSCF would increase engraftment of human HSCs and human cell chimerism in nonirradiated adult mice. Six- to 10-week-old nonirradiated NSG and NSG-Tg(hu-mSCF) mice were engrafted with 3 × 104 T cell–depleted CD34+ cord blood-derived HSCs.24 Twelve weeks later, flow cytometry was used to determine the levels of human CD45+ cell chimerism.
Surprisingly, we observed that nonirradiated adult NSG mice consistently exhibited human CD45+ cell chimerism in the spleen (Table 1), blood (supplemental Table 3), and bone marrow (supplemental Table 4) 12 weeks after injection with human HSCs. Chimerism levels of human CD45+ cells in nonirradiated adult NSG mice were equivalent to that observed in nonirradiated adult NSG-Tg(hu-mSCF). In spleen and blood of both NSG and NSG-Tg(hu-mSCF) mice, the predominant human cell population was composed of CD20+ B cells with very low levels of human CD3+ T cells (Table 1; supplemental Table 3). There was also a significant increase in the percentages of CD71+235a+ erythroid progenitor cells in NSG-Tg(hu-mSCF) compared with NSG mice (supplemental Table 4). In addition, in both nonirradiated HSC-engrafted NSG and NSG-Tg(hu-mSCF) mice, normal ratios of developing human thymocyte subsets (CD4+CD8+, CD4+CD8−, and CD4−CD8+) were observed (supplemental Table 5), although only low levels of human T cells were detectable in the periphery (Table 1; supplemental Tables 3-4).
Transgenic expression of human membrane-bound SCF enhances human hematopoietic cell chimerism in HSC-engrafted newborn NSG mice
Blood chimerism.
We previously demonstrated that engraftment of human HSCs into irradiated newborn mice leads to heightened levels of human T cells compared with HSC engraftment into irradiated adults.24 To determine whether nonirradiated newborn NSG-Tg(hu-mSCF) mice support equivalent engraftment of human HSCs and development of human T cells compared with irradiated NSG mice, we transplanted 4 groups of newborn mice with human HSCs: (1) nonirradiated NSG-Tg(hu-mSCF), (2) nonirradiated NSG, (3) 100-cGy irradiated NSG-Tg(hu-mSCF), and (4) 100 cGy irradiated NSG.
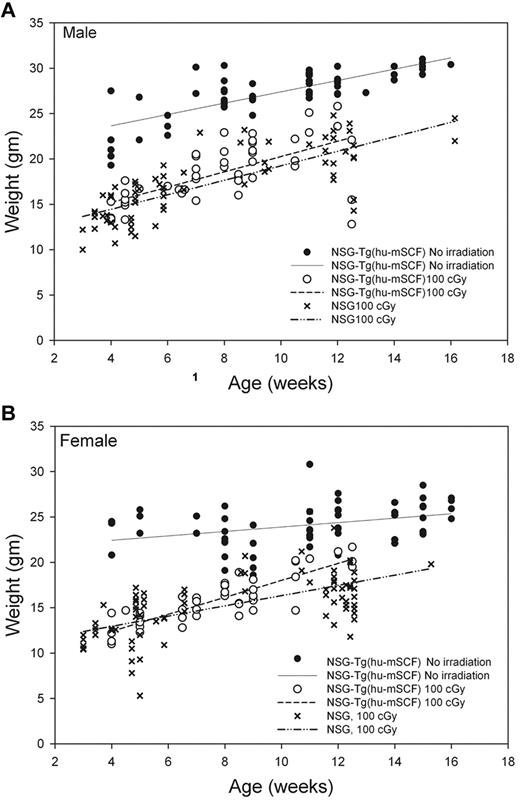
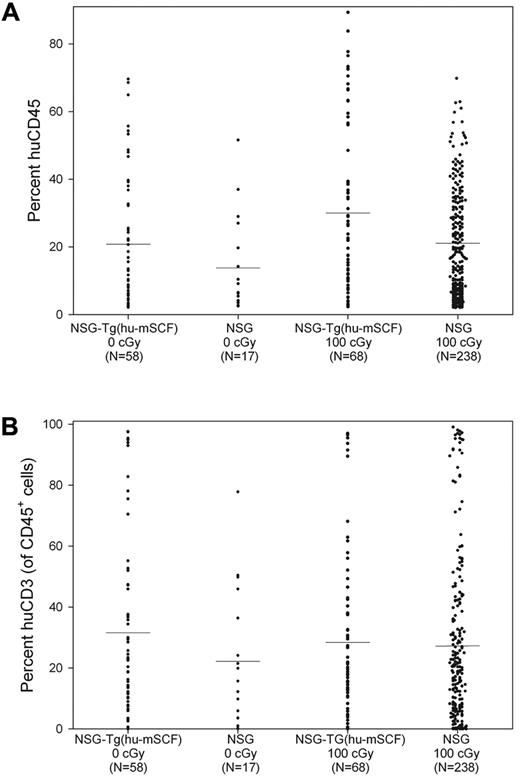
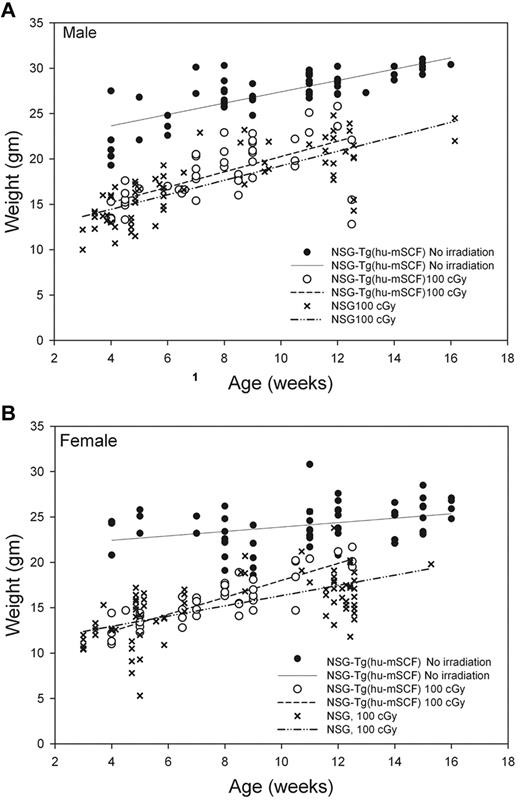
We first observed that nonirradiated HSC-engrafted NSG-Tg(hu-mSCF) mice gained weight with faster kinetics and attained higher adult weights than did irradiated HSC-engrafted NSG or NSG-Tg(hu-mSCF) mice (Figure 2). The weights of nonirradiated HSC-engrafted NSG-Tg(hu-mSCF) mice were equivalent to the weights of nonirradiated, non-HSC–engrafted NSG mice in our colony (data not shown). The weights attained by irradiated, HSC-engrafted NSG-Tg(hu-mSCF) mice were equivalent to those attained by irradiated HSC-engrafted NSG mice and lower than that observed in nonirradiated mice (Figure 2). We next observed that the frequency of mice with more than 2% human CD45+ cell chimerism in the blood was equivalent between nonirradiated NSG (74%, 17 of 23), irradiated NSG (83%, 238 of 286), nonirradiated NSG-Tg(hu-mSCF) (70%, 58 of 82), and irradiated NSG-Tg(hu-mSCF) (72%, 68 of 94) mice. In addition, the percentage of human CD45+ cell chimerism in the blood of each group was also equivalent (Figure 3; Table 2). Human CD3+ T cells developed in each group, and the percentage of CD45+ cells that were CD3+ in the blood was equivalent (Figure 3; Table 2). Investigation of the B lineage, myeloid/granulocyte, dendritic cell, and monocyte/macrophage cell lineages revealed no significant differences in human cell subsets as a percentage of human CD45+ cells among different groups (Table 2).
Weights of mice engrafted with human HSC as newborns. Weights of nonirradiated NSG-Tg(hu-mSCF) and 100-cGy irradiated NSG and NSG-Tg(hu-mSCF) mice engrafted with human HSCs as newborns were obtained over time after weaning. (A) Male mice. (B) Female mice. Each symbol represents an individual data point. The lines represent the average of the individual data points.
Weights of mice engrafted with human HSC as newborns. Weights of nonirradiated NSG-Tg(hu-mSCF) and 100-cGy irradiated NSG and NSG-Tg(hu-mSCF) mice engrafted with human HSCs as newborns were obtained over time after weaning. (A) Male mice. (B) Female mice. Each symbol represents an individual data point. The lines represent the average of the individual data points.
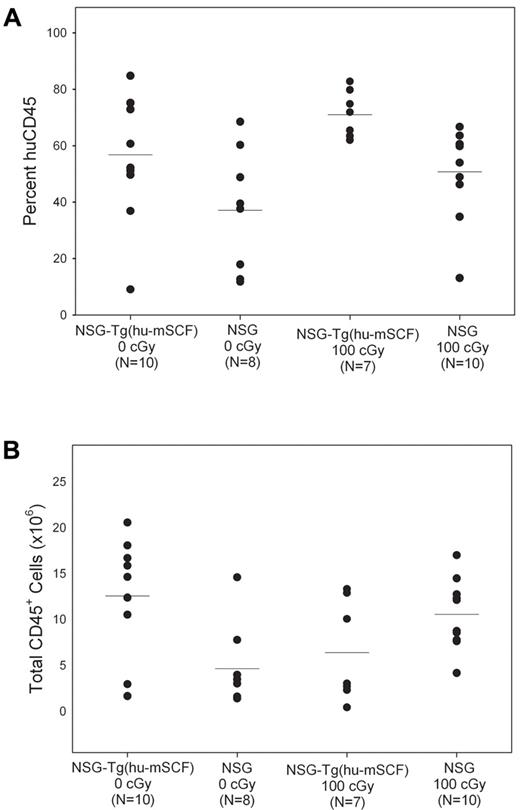
Human CD45+ cell chimerism and CD3+ T-cell development in the blood of newborn-engrafted mice at 12 weeks of age. Nonirradiated NSG and NSG-Tg(hu-mSCF) and 100-cGy irradiated NSG and NSG-Tg(hu-mSCF) newborn mice were engrafted with human HSCs as described in “Engraftment of mice human HSCs.” Mice were analyzed by flow cytometry for the percentage of human CD45+ and the percentage of human CD45+ cells that were CD3+ in the blood at 12 weeks of age. (A) Percentage of human CD45+ cells. (B) Percentage of human CD45+ cells that were CD3+. No significant difference between groups was observed.
Human CD45+ cell chimerism and CD3+ T-cell development in the blood of newborn-engrafted mice at 12 weeks of age. Nonirradiated NSG and NSG-Tg(hu-mSCF) and 100-cGy irradiated NSG and NSG-Tg(hu-mSCF) newborn mice were engrafted with human HSCs as described in “Engraftment of mice human HSCs.” Mice were analyzed by flow cytometry for the percentage of human CD45+ and the percentage of human CD45+ cells that were CD3+ in the blood at 12 weeks of age. (A) Percentage of human CD45+ cells. (B) Percentage of human CD45+ cells that were CD3+. No significant difference between groups was observed.
Bone marrow chimerism.
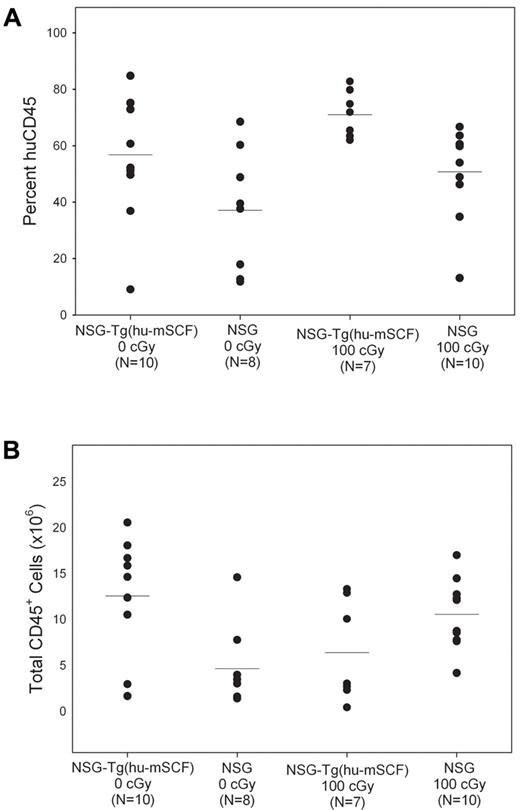
The mean percentages of human CD45+ cells in the bone marrow of the 4 groups of newborn HSC-engrafted mice varied between 40% and 70% (Figure 4). The absolute numbers of human CD45+ cells in the bone marrow of 100-cGy irradiated NSG mice and nonirradiated NSG-Tg(hu-mSCF) were similar (P = not significant), and both were significantly higher than that observed in nonirradiated NSG mice (Table 3). There was also a significant increase in the percentages of CD71+CD45− and CD71+235a+ erythroid progenitor cells in nonirradiated NSG-Tg(hu-mSCF) compared with irradiated or nonirradiated NSG mice (Table 3). No significant differences were observed in the overall chimerism of human hematopoietic progenitor cells between the 4 groups (Table 3) or in the hematopoietic potential of CD34+/CD38− HSCs purified from nonirradiated NSG-Tg(hu-mSCF) or irradiated NSG mice as determined by colony-forming cell assay (supplemental Figure 1, 71 ± 30 colonies/1000 cells and 78 ± 21 colonies/1000 cells, respectively, P = .86).
Human CD45+ cell chimerism in the bone marrow of newborn-engrafted mice at 12 weeks of age. Nonirradiated NSG and NSG-Tg(hu-mSCF) and 100-cGy irradiated NSG and NSG-Tg(hu-mSCF) newborn mice were engrafted with human HSCs as described in “Engraftment of mice human HSCs.” Mice were analyzed by flow cytometry for the percentage of human CD45+ cells in the bone marrow at 12 weeks of age. (A) Percentage of human CD45+ cells. (B) Total number of human CD45+ cells. Each symbol represents an individual animal. Significant differences were observed in the number of human CD45+ cells: 0 cGy NSG-Tg(hu-mSCF) vs 0 cGy NSG (P < .01); 0 cGy NSG vs 100 cGy NSG (P < .01).
Human CD45+ cell chimerism in the bone marrow of newborn-engrafted mice at 12 weeks of age. Nonirradiated NSG and NSG-Tg(hu-mSCF) and 100-cGy irradiated NSG and NSG-Tg(hu-mSCF) newborn mice were engrafted with human HSCs as described in “Engraftment of mice human HSCs.” Mice were analyzed by flow cytometry for the percentage of human CD45+ cells in the bone marrow at 12 weeks of age. (A) Percentage of human CD45+ cells. (B) Total number of human CD45+ cells. Each symbol represents an individual animal. Significant differences were observed in the number of human CD45+ cells: 0 cGy NSG-Tg(hu-mSCF) vs 0 cGy NSG (P < .01); 0 cGy NSG vs 100 cGy NSG (P < .01).
Spleen chimerism.
The percentages of human CD45+ cells in the spleens of the 4 groups of newborn HSC-engrafted mice were similar at 12 weeks of age, but the absolute numbers of human CD45+ cells in the spleen of nonirradiated NSG-Tg(hu-mSCF) mice were significantly higher (∼ 2.5-fold higher) than that observed in nonirradiated NSG mice (Table 4). No significant differences were observed with the development of human immune cell subsets between the groups.
Thymus chimerism.
In the nonirradiated NSG-Tg(hu-mSCF) mice, normal ratios of developing human thymocyte subsets (CD4+CD8+, CD4+CD8−, and CD4−CD8+) cells were observed (Table 5).
Human HSC-engrafted newborn NSG-Tg(hu-mSCF) mice develop a functional human immune system
In tissues and organs examined in the 4 groups of mice, lineages of all cell phenotypes (T, B, myeloid, dendritic, and erythroid) were observed, suggesting that, even in the nonirradiated HSC-engrafted mice, a full human hematopoietic and immune system was generated (Tables 2,Table 3,Table 4–5).
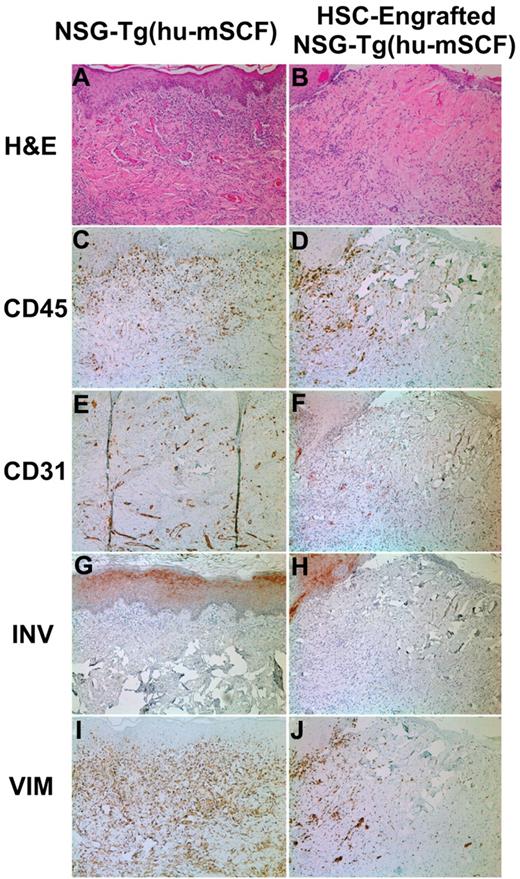
To test whether the phenotypic development of a human immune system resulted in a functional human immune system, we transplanted histoincompatible human skin grafts on human HSC-engrafted mice. Eleven nonirradiated newborn NSG-Tg(hu-mSCF) mice were engrafted with HSCs and transplanted with human skin grafts between 12 and 16 weeks of age as described in “Skin transplantation protocol.” At 4 to 5 weeks after skin transplantation, all 11 mice demonstrated various stages of rejection of the skin graft, with 5 showing complete rejection at this time point (Figure 5 right column). In contrast, in NSG-Tg(hu-mSCF) mice not engrafted with HSCs (N = 6), all skin grafts healed and displayed no signs of rejection (Figure 5 left column). These data indicate that the human immune system generated after engraftment of HSCs into nonirradiated newborn NSG-Tg(hu-mSCF) mice is functional.
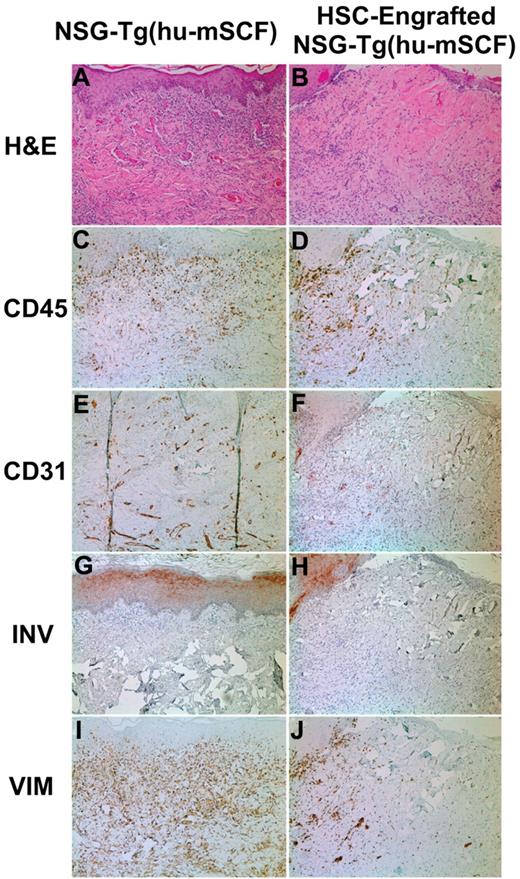
Skin transplantation on nonirradiated NSG-Tg(hu-mSCF) mice that were non-HSC–engrafted or engrafted with human HSCs as newborns. Nonirradiated NSG-Tg(hu-mSCF) mice were non-HSC–engrafted (left panels) or engrafted with human HSCs as newborns (right panels) as described in “Engraftment of mice with human HSCs.” At 12 to 14 weeks of age, all mice were transplanted and monitored for rejection of human skin allografts. Left side indicates the stain used for each row. (A-B) H&E indicates hematoxylin and eosin. (C-D) CD45 indicates all human hematolymphoid cells. (E-F) CD31 indicates human endothelium. (G-H) INV indicates human involucrin. (I-J) VIM indicates human vimentin.
Skin transplantation on nonirradiated NSG-Tg(hu-mSCF) mice that were non-HSC–engrafted or engrafted with human HSCs as newborns. Nonirradiated NSG-Tg(hu-mSCF) mice were non-HSC–engrafted (left panels) or engrafted with human HSCs as newborns (right panels) as described in “Engraftment of mice with human HSCs.” At 12 to 14 weeks of age, all mice were transplanted and monitored for rejection of human skin allografts. Left side indicates the stain used for each row. (A-B) H&E indicates hematoxylin and eosin. (C-D) CD45 indicates all human hematolymphoid cells. (E-F) CD31 indicates human endothelium. (G-H) INV indicates human involucrin. (I-J) VIM indicates human vimentin.
Poor human hematopoietic cell chimerism in nonirradiated newborn NOD-Rag1null IL2rγnull (NRG)-Tg(hu-mSCF) mice after engraftment with human HSCs
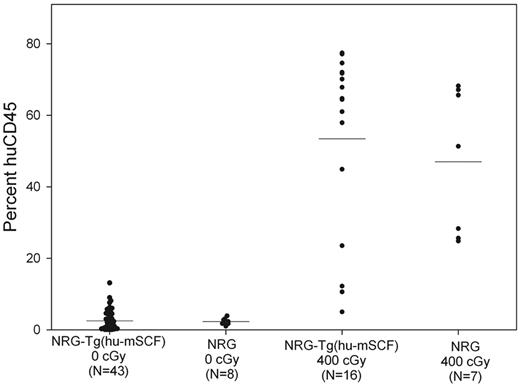
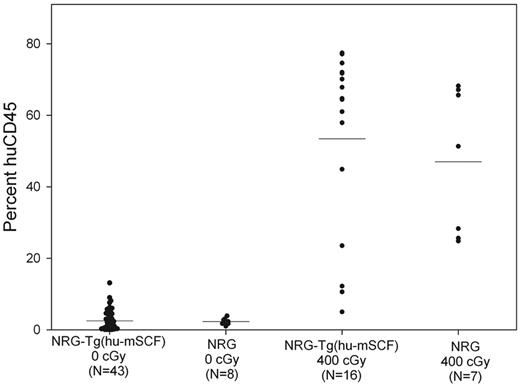
To determine whether human cell chimerism in nonirradiated newborn NSG-Tg(hu-mSCF) mice required the scid mutation, we generated NOD-Rag1null IL2rγnull (NRG)-Tg(hu-mSCF) mice and engrafted nonirradiated newborns with human HSCs. As shown in Figure 6, nonirradiated newborn NRG-Tg(hu-mSCF) and NRG mice engrafted with human HSCs exhibited poor human CD45+ cell chimerism at 12 weeks of age in their blood. In addition, human cell chimerism in the spleen and bone marrow was also very low in nonirradiated NRG-Tg(hu-mSCF) and NRG mice (data not shown). In contrast and as expected,29 400-cGy irradiated newborn NRG-Tg(hu-mSCF) and NRG mice engrafted with the same cord blood-derived human HSCs generated high levels of human CD45+ cells at 12 weeks of age in all tissues examined (Figure 6).
Human CD45+ cell chimerism in the blood of newborn-engrafted mice at 12 weeks of age. Nonirradiated NRG and NRG-Tg(hu-mSCF) mice and 400-cGy NRG and NRG-Tg(hu-mSCF) mice were engrafted as newborns with human HSCs as described in “Engraftment of mice with human HSCs.” Mice were analyzed by flow cytometry for the percentage of human CD45+ cells in the blood at 12 weeks of age. Each symbol represents an individual animal. No significant differences were observed among the nonirradiation groups or among the irradiated groups. Engraftment in either of the nonirradiated groups was significantly different from the engraftment observed in either of the irradiated groups (P < .001).
Human CD45+ cell chimerism in the blood of newborn-engrafted mice at 12 weeks of age. Nonirradiated NRG and NRG-Tg(hu-mSCF) mice and 400-cGy NRG and NRG-Tg(hu-mSCF) mice were engrafted as newborns with human HSCs as described in “Engraftment of mice with human HSCs.” Mice were analyzed by flow cytometry for the percentage of human CD45+ cells in the blood at 12 weeks of age. Each symbol represents an individual animal. No significant differences were observed among the nonirradiation groups or among the irradiated groups. Engraftment in either of the nonirradiated groups was significantly different from the engraftment observed in either of the irradiated groups (P < .001).
Discussion
We have shown that transgenic expression of human membrane-bound SCF enhances human HSC engraftment in nonirradiated newborn NSG mice. Although nonirradiated newborn and adult NSG mice engraft at low levels with human HSCs, transgenic expression of hu-mSCF enhanced engraftment in nonirradiated newborn mice. The immune system that developed in nonirradiated newborn HSC-engrafted NSG-Tg(hu-mSCF) mice was functional as evidenced by their ability to reject human skin allografts. The enhanced human cell chimerism was dependent on the scid mutation as nonirradiated HSC-engrafted newborn NRG-Tg(hu-mSCF) mice did not support high-level chimerism. The availability of NSG-Tg(hu-mSCF) mice now permits engraftment of human HSC in the absence of irradiation preconditioning.
Sublethal irradiation of newborn mice resulted in deficient weight gain. It is also known that sublethal irradiation causes damage to neurons in the developing brain.30 Such effects of irradiation complicate interpretation of studies in regenerative medicine. For example, radiation damage of newborn mice increases numbers of binucleated heterokaryons consisting of Purkinje cells fused to bone marrow–derived cells.31 We hypothesized that transgenic expression of human membrane-bound SCF in the absence of transgenic expression of human GM-CSF as in the NOD-scid triple transgenic stock17 would enhance human HSC engraftment and permit engraftment of human HSCs without preconditioning irradiation. To test this, we backcrossed the Tg(hu-mSCF) transgene onto the NSG strain and characterized this strain of mice and its ability to support human HSC engraftment.
We first observed that transgenic expression of human mSCF induced only minor alterations in the hematologic profile of NSG mice. There was a significant increase in reticulocyte numbers and percentages, suggesting that a mild anemia was being corrected. NSG mice have not previously been reported to exhibit anemia, and in our original report on NSG mice, this parameter was not evaluated.23 However, we did observe in the original studies a lower packed red cell volume in NSG mice compared with NOD-scid mice,23 suggesting, and confirmed in this report, that NSG mice may have a mild anemia.
Surprisingly, we observed that both adult NSG and newborn NSG mice could be engrafted with human HSCs in the absence of preconditioning irradiation. We further observed that transgenic expression of human membrane-bound SCF enhanced the engraftment of the human HSC in newborn nonirradiated recipients. As we have observed in HSC-engrafted irradiated adult NSG recipients,24 the generation of human T cells in nonirradiated adult recipients was poor, irrespective of whether the human mSCF transgene was expressed. We have proposed that the lack of robust human T-cell development in adult NSG mice may be a consequence of the development of thymic cysts as the mouse ages.24 The poor T-cell generation from HSC is probably the result of the presence of a poor supportive thymic microenvironment for human stem cell differentiation into mature T cells. Based on this initial observation, all subsequent studies were performed on newborn NSG and NSG-Tg(hu-mSCF) mice.
In nonirradiated HSC-engrafted newborn NSG-Tg(hu-mSCF) mice, we observed that higher numbers of human CD45+ cells were consistently generated compared with similarly engrafted nonirradiated newborn NSG mice. The absolute numbers of human CD45+ cells in the nonirradiated HSC-engrafted newborn NSG-Tg(hu-mSCF) mice were comparable with that observed in irradiated newborn NSG mice, suggesting that transgenic expression of human mSCF enhanced human HSC engraftment up to the level achieved in irradiated newborn NSG mice but without the need for irradiation preconditioning. It has been suggested that the human SCF can lead to occupancy of the murine c-kit receptor but that signaling is suboptimal.21 This would lead to a selective advantage of the human HSCs after engraftment. Another important feature of nonirradiated HSC-engrafted newborn NSG-Tg(hu-mSCF) mice was their robust growth, higher than that achieved in irradiated newborn human HSC-engrafted NSG and NSG-Tg(mSCF) mice. This may be an important advantage of using nonirradiated newborn HSC-engrafted NSG mice as we have observed that human HSC-engrafted irradiated NSG mice with low body weights are highly susceptible to adverse outcomes after traumatic surgical procedures, such as human skin or islet transplantation.
Nonirradiated HSC-engrafted newborn NSG-Tg(hu-mSCF) mice were able to generate robust numbers of human CD20+ B cells as well as cells representative of all hematopoietic lineages evaluated and, in contrast to adult recipients, also generated robust numbers of human CD3+ T cells. The human immune system generated in these mice appears to be functional as evidenced by its ability to recognize histoincompatible human skin grafts and mount a rejection to this graft. The transplanted human skin was genetically allogeneic to the developing human immune system. However, T cells from HSC-engrafted NSG mice are thought to develop in the murine thymus on H2-MHC; therefore, the human skin grafts would be considered histoincompatible in terms of antigen recognition. Graft rejection in HSC-engrafted mice occurred with delayed kinetics (4 weeks for complete rejection) compared with graft rejection in nonimmunosuppressed humans or in immunocompetent mice that generally occurs within 2 weeks. Understanding the mechanisms underlying the delayed graft rejection may permit modification of the immunodeficient recipient to enhance further human immune cell engraftment and function.
To determine whether the ability to engraft immunodeficient mice without irradiation required both the scid and IL2rγnull genetic mutation, we generated NRG-Tg(hu-mSCF) mice and tested the ability of irradiated newborn and nonirradiated newborn mice to be engrafted with human HSCs. The scid mutation results in defective double-stranded DNA repair and concomitant increased radiosensitivity,32-34 whereas neither the Rag1- nor Rag2-targeted mutations cause defective DNA repair.35,36 Surprisingly, we observed that human cell chimerism in nonirradiated mice was highly dependent on the scid gene as chimerism levels in nonirradiated newborn NRG-Tg(hu-mSCF) and NRG mice were low. This was not the result of the specific cord HSCs used in these experiments as engraftment of irradiated NRG and NRG-Tg(hu-mSCF) mice with aliquots of these same HSC was, as expected,24,25 robust. This suggests that generation of human mSCF transgenic mice on stocks of immunodeficient mice based on mutations in the Rag1 or Rag2 genes, such as the BALB/c-Rag2null IL2rγnull strain of mice, will not permit robust engraftment of HSCs in the absence of irradiation preconditioning.
The scid mutation causes a defect in double-stranded DNA repair.37 The ability of NSG, but not NRG, mice to support human HSC engraftment is probably associated with this repair defect and with the effect of the scid mutation on bone marrow niche occupancy. It has recently been reported that the scid, but not the Rag1, mutation results in loss of long-term bone marrow niche occupancy and confers heightened engraftment of mouse HSCs.38 Mutations in mice at the kit locus encoding the receptor for SCF and at the Steel locus encoding the kit ligand result in greatly increased radiosensitivity.39,40
Limitations of human immune function, in addition to those noted during the rejection of human skin grafts, remain in these human HSC-engrafted mouse model systems. For example, poor lymph node and splenic structures are observed in human HSC-engrafted immunodeficient mice,5,41,42 which did not appear to be corrected by transgenic expression of hu-mSCF. Poor human erythropoiesis in nonirradiated HSC-engrafted newborn NSG-Tg(hu-mSCF) mice is also observed as was observed in other studies,43,44 although we did detect an increase in bone marrow erythroid progenitor cells in nonirradiated NSG-Tg(hu-mSCF) compared with irradiated or nonirradiated NSG mice. These data suggest that the transgenic expression of human mSCF enhances human erythropoiesis in the bone marrow of the recipients. The inability of human red blood cells to circulate in engrafted NSG mice may be the result of their expression of human CD47 on the red blood cells. Inhibition of phagocyte activity depends on ligation of macrophage-expressed SIRP-α by CD47.45 However, in the case of mouse macrophages and human red blood cells, the human CD47 would be recognized as “foreign.” Although NOD mice express a SIRP-α polymorphism very similar to that of human,46 it may still be foreign enough to fail to inhibit recognition and phagocytosis by the mouse macrophages. Finally, generation of human NK cells is increased in human HSC-engrafted mice expressing high levels of human IL-15,47,48 but full NK cell function is not achieved. Development of HLA-transgenic mice expressing the appropriate HLA molecule to permit “licensing” of NK cells49 may enhance the generation of functional NK cells in human immune system engrafted mice treated with human IL-15.
In conclusion, we have shown that newborn NSG-Tg(hu-mSCF) mice can be engrafted with human HSCs in the absence of irradiation preconditioning. The level of human cell chimerism achieved is comparable with that observed in irradiated NSG mice in the absence of transgenic expression of human mSCF. Nonirradiated human HSC-engrafted newborn NSG-Tg(hu-mSCF) mice exhibit robust body weight growth, and the immune system that develops is functional and capable of rejecting human skin allografts. These mice represent a new model of human HSC-engrafted mice that can be generated without the need for irradiation and exhibit engraftment characteristics similar to those achieved in irradiated NSG mice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Linda Paquin, Pamela Wooton, Allison Ingalls, Michelle Farley, and Rebecca Riding for excellent technical assistance and David Williams for sending C3H/HeJ mice carrying the human SCF transgene.
This work was supported by the National Institutes of Health (research grants AI46629, AI050864, AI083911, AI073871, HL077642, CA34196, and DK089572), the Diabetes Endocrinology Research Center (institutional grant DK32520), the University of Massachusetts Center for AIDS Research (grant P30 AI042845), the Juvenile Diabetes Research Foundation, International, the Helmsley Foundation, and US Army Medical Research Institute for Infectious Diseases.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: M.A.B. and W.J.R. designed and performed research, wrote the manuscript, and analyzed data; J.L., L.B., A.W., and B.G. performed research and analyzed data; V.H. designed research and wrote the manuscript; M.H., R.I., and R.D. provided reagents and reviewed the manuscript; L.D.S. designed and performed research, contributed new reagents, analyzed data, and wrote the manuscript; and D.L.G. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Brehm, Department of Molecular Medicine, University of Massachusetts Medical School, 373 Plantation St, Biotech 2, Suite 218, Worcester, MA 01605; e-mail: michael.brehm@umassmed.edu.