To the editor:
Kumari and colleagues have recently reported a detailed analysis of BCR-ABL expression in CFU-Cs (colony forming units in culture) of patients with chronic myeloid leukemia (CML).1 Using quantitative reverse-transcription PCR on individual Ph1 hematopoietic colonies, they demonstrated that CFU-Cs from patients in major molecular response (MMR) displayed lower BCR-ABL mRNA expression than CFU-Cs from patients at diagnosis. In addition, the authors observed a large variability of BCR-ABL/ABL ratios in homogeneous subpopulations of CFU-Cs. These interesting findings were evaluated in the progenitor compartment and not in the more primitive stem cell compartment, including long-term-culture initiating cell (LTC-IC)–derived CFU-Cs.
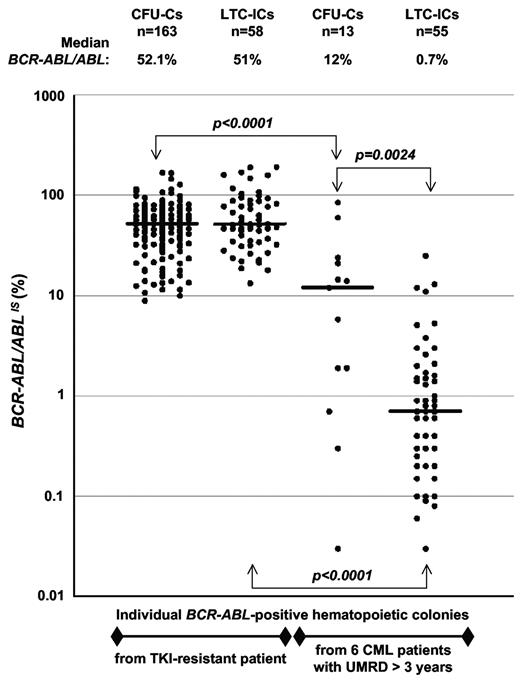
We have previously analyzed BCR-ABL–expressing leukemic progenitors and stem cells, either in the context of imatinib resistance2 or that of sustained undetectable molecular residual disease (UMRD).3 In the light of the data presented by Kumari et al, we wished to examine the BCR-ABL mRNA levels in individual progenitors and LTC-IC derived CFU-Cs from our experiments. In the first study of a patient with imatinib-resistant CML,2 BCR-ABL/ABL ratios in CFU-Cs and LTC-IC-derived CFU-Cs were found to be similar, with a median value of 52.1% and 51% respectively, comparable with the ratio (68%) measured in peripheral blood (Figure 1). In the second work,3 we have analyzed the BCR-ABL mRNA expression in 6 patients with sustained UMRD (> 3 years). We have detected persistent BCR-ABL–expressing stem cells not only in the patient on targeted therapy, but also in the other 5 patients, in whom IFN-α or imatinib treatments were discontinued for 2-13 years. The analysis of BCR-ABL/ABL ratios in hematopoietic colonies showed a large expression variability from one CFU-C to another, in accordance with Kumari et al.1 Moreover, a significant difference was observed between the amount of BCR-ABL mRNA transcript in CFU-Cs and LTC-IC–derived progeny in the context of UMRD (Figure 1). Interestingly, primitive stem cells expressed significantly less BCR-ABL mRNA than committed progenitors (median BCR-ABL/ABL ratios of 0.7% and 12%, respectively).
BCR-ABL expression in individual BCR-ABL-positive hematopoietic colonies. The determination of BCR-ABL/ABL ratios was performed according to the international scale (IS) standardization by quantitative RT-PCR on individual hematopoietic colonies from CFU-C (colony forming unit-cell) and LTC-IC (long-term culture-initiating cell) assays. Dots represent BCR-ABL/ABL ratios for single colonies (CFU-Cs or LTC-ICs) from a patient resistant to tyrosine kinase inhibitor therapy and from 6 patients with undetectable molecular residual disease (UMRD) for more than 3 years. Mann Whitney U tests were used to estimate differences between groups. Significant differences are reported after Bonferroni correction to account for multiple testing. The horizontal lines indicate median BCR-ABL/ABL ratios, and the number of BCR-ABL–positive CFU-Cs and LTC-ICs analyzed in the study is shown.
BCR-ABL expression in individual BCR-ABL-positive hematopoietic colonies. The determination of BCR-ABL/ABL ratios was performed according to the international scale (IS) standardization by quantitative RT-PCR on individual hematopoietic colonies from CFU-C (colony forming unit-cell) and LTC-IC (long-term culture-initiating cell) assays. Dots represent BCR-ABL/ABL ratios for single colonies (CFU-Cs or LTC-ICs) from a patient resistant to tyrosine kinase inhibitor therapy and from 6 patients with undetectable molecular residual disease (UMRD) for more than 3 years. Mann Whitney U tests were used to estimate differences between groups. Significant differences are reported after Bonferroni correction to account for multiple testing. The horizontal lines indicate median BCR-ABL/ABL ratios, and the number of BCR-ABL–positive CFU-Cs and LTC-ICs analyzed in the study is shown.
The persistence of primitive leukemic stem cells in CML patients with UMRD has been recently documented,3,4 demonstrating that, despite a major success in the eradication of bulk leukemic cells, imatinib therapy does not fully eradicate leukemic stem cells. Several biologic explanations for the intrinsic refractoriness of CML stem cells against imatinib have been proposed,5,6 but a mechanism involving low BCR-ABL expression in these primary cells had not been suggested before. The variable BCR-ABL transcription by Ph1 progenitors has previously been described in patients with chronic phase CML.7,8 Kumari and coworkers observed a lower BCR-ABL expression in individual hematopoietic progenitor colonies from patients with MMR, and suggested a relationship between this phenomenon and the persistence of leukemic stem cells resistant to imatinib. Our data, based on patients with sustained UMRD, show that low amounts of BCR-ABL mRNA transcripts are observed not only in hematopoietic progenitors but also in primitive stem cells with a major reduction in the latter. Therefore, the weak level of BCR-ABL expression in persistent leukemic stem cells could be an additional mechanism explaining their innate resistance toward imatinib and perhaps to other tyrosine kinase inhibitors. In addition, in imatinib discontinuation context, correlation studies between levels of BCR-ABL transcripts in residual leukemic stem cells and molecular relapse could be of major interest.
Authorship
Acknowledgments: This work was supported by grants from INCa (Institut National du Cancer), Cancéropôle Grand Ouest, Université de Poitiers, Inserm, the association Laurette Fugain (Project ALF 09-04), and a supplementary contribution from Novartis Pharma SAS France.
Contribution: J.C.C. and N.S. performed experiments; J.G. computerized expression data and performed statistical analyses; F.G. collected and analyzed clinical data; J.C.C. and A.G.T. analyzed the data and wrote the manuscript; and all authors read and approved the manuscript in its final version.
Conflict-of-interest disclosure: F.G. has received consulting fees and research funding from Novartis Oncology and Bristol Myers Squibb. A.G.T has received lecture fees and research funding from Novartis Oncology and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Dr J. C. Chomel, Service d'Hématologie et Oncologie Biologique–Inserm U935, CHU de Poitiers, 2 rue de la Milétrie, 86021 Poitiers Cedex, France; e-mail: j.c.chomel@chu-poitiers.fr.